ABCSG Trial: Survival Benefit for Tamoxifen → Anastrozole
ABCSG Trial: Survival Benefit for Tamoxifen. Anastrozole Updated results from Austrian Breast and Colorectal Cancer Study Group Trial 8 confirmed a survival difference for the sequencing strategy of tamoxifen followed by anastrozole (Arimidex), compared to 5 years of tamoxifen (abstract 14). Preliminary results (median follow-up 55 mo) had previously revealed a 24% reduction in recurrence in favor of the sequencing strategy, although the difference was not statistically significant.
ABCSG Trial: Survival Benefit for Tamoxifen → Anastrozole Updated results from Austrian Breast and Colorectal Cancer Study Group Trial 8 confirmed a survival difference for the sequencing strategy of tamoxifen followed by anastrozole (Arimidex), compared to 5 years of tamoxifen (abstract 14). Preliminary results (median follow-up 55 mo) had previously revealed a 24% reduction in recurrence in favor of the sequencing strategy, although the difference was not statistically significant.
Raimund Jakesz, MD, presented mature results of Trial 8, involving 3,714 postmenopausal women with newly diagnosed hormone-sensitive early breast cancer. At a median follow-up of 72 months, a sequencing strategy of 2 years of tamoxifen followed by 3 years of anastrozole improved recurrence-free survival (RFS) vs 5 years of tamoxifen. With the sequencing approach, RFS events were reduced by 21% (P = .038) regardless of whether patients actually received the assigned treatment, and by 27% (P = .001) in patients who received treatment as planned.
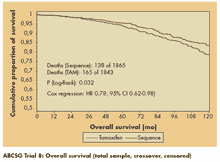
Patients with sequential endocrine treatment also had a significant improvement in overall survival (see figure). In the intent-to-treat analysis of all patients, deaths were significantly reduced by 22% (P = .032). Higher levels of estrogen receptor or progesterone receptor positivity were predictive of longer RFS and overall survival, the risks of which were reduced by approximately 35% with sequencing, Dr. Jakesz reported.
“Switching from tamoxifen to anastrozole after 2 years of tamoxifen significantly improves recurrence-free and overall survival,” he concluded. “This trial clearly shows a survival benefit over 5 years of adjuvant tamoxifen.”