The management of rectal cancer in patients with metastatic disease at presentation is highly variable. There are no phase III trials addressing therapeutic approaches, and the optimal sequencing of chemotherapy, radiation therapy, and surgery remains unresolved. Although chemoradiation is standard for patients with stage II/III rectal cancer, its role in the metastatic setting is controversial. Omitting chemoradiation may not be appropriate in all stage IV patients, particularly those with symptomatic primary tumors. Moreover, outcomes in this setting are vastly different, as some treatments carry the potential for cure in selected patients, while others are purely palliative. The American College of Radiology Appropriateness Criteria are evidence-based guidelines for specific clinical conditions that are reviewed every 3 years by a multidisciplinary expert panel. The guideline development and review include an extensive analysis of current medical literature from peer-reviewed journals and the application, by the panel, of a well-established consensus methodology (Modified Delphi) to rate the appropriateness of imaging and treatment procedures. In instances in which evidence is lacking or not definitive, expert opinion may be used as the basis for recommending imaging or treatment.
Summary of Literature Review
Introduction/Background
According to the American Cancer Society, 40,000 new cases of rectal cancer were diagnosed in the United States in 2014.[1] Approximately 15% of these patients had metastatic disease at presentation.[2] The management of metastatic colorectal cancer (mCRC) has evolved over the past several decades with the introduction of improved surgical techniques, radiologic and pathologic staging, and regimens for systemic and radiation therapy (RT). As a result, the overall survival of patients with mCRC has improved significantly in recent years.[3] Furthermore, a small but important group of these patients potentially may be cured of their disease through multimodality management.[4] However, for the majority of patients with mCRC, the aim of therapy is to prolong survival and palliate symptoms.
Management of patients with newly diagnosed metastatic rectal cancer (mRC) may be complex, and treatment decisions benefit from multidisciplinary input. Management must be individualized based on the overall medical condition of the patient, the extent and distribution of metastatic disease, and the patient’s wishes.
Management of the Primary Tumor
The optimal management of the primary tumor in patients with metastatic disease is controversial; however, the paradigm is changing with the substantial improvements in systemic therapy and the expected duration of survival. Given the potential for cure after resection of all locoregional and distant disease, the approach to the primary tumor is determined by the resectability of the metastatic lesions as well as the severity of symptoms from the primary rectal mass.
Resectable Metastatic Disease
After resection of the primary tumor and distant metastases, patients with mCRC may experience long-term survival, and a small subset may be cured.[4] Therefore, aggressive surgical management is warranted.
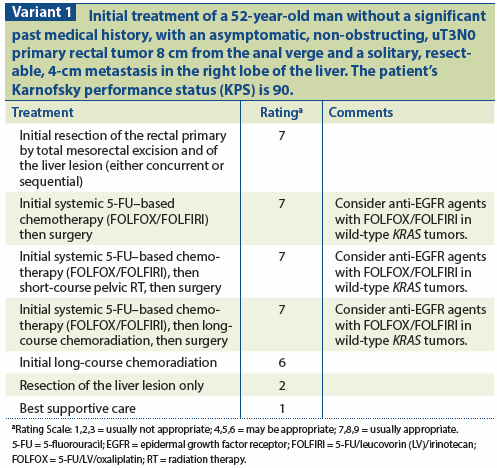
Patients with low-volume, stage T1-T2N0 metastatic disease, or high rectal primary tumors may be treated ideally with upfront resection of the primary tumor and metastases or with preoperative chemotherapy alone followed by a synchronous or staged resection of the primary tumor and metastases. On the other hand, patients with T3-4, regional node-positive or low-lying primary tumors should be considered for preoperative combined-modality therapy (CMT) with 5-fluorouracil (5-FU) and pelvic RT to reduce the risk of pelvic recurrence. Although limited data exist to support this approach in mRC, the improved local control and decreased toxicity with preoperative vs postoperative CMT may be extrapolated from the data on patients with locally advanced rectal cancer.[5] In the United States, long-course chemoradiation (50.4 Gy in 28 fractions) is the standard preoperative management of rectal cancer; however, short-course RT (25 Gy in 5 fractions) may be considered in the setting of mRC to reduce the delay before surgery and initiation of full-dose systemic therapy.[6] Any patient with an obstructing tumor should undergo surgical diversion prior to initiating CMT, regardless of the fractionation schedule used. A less preferable option for these patients would be endoscopic placement of a rectal stent.
Patients who have undergone upfront complete resection of both the primary tumor and all known metastatic disease can be considered candidates for the postoperative management routinely provided in stage II or III rectal cancer, which may include adjuvant chemotherapy with or without chemoradiation based on the stage and location of the primary tumor. Postoperative CMT should be strongly considered for any patient with T4 disease who did not receive preoperative pelvic RT.
Unresectable Metastatic Disease
The primary management of unresectable metastatic disease is chemotherapy. In the majority of cases, initiation of chemotherapy should not be postponed in favor of local therapy, given the high response rates and infrequency of rapid progression through first-line regimens. One important exception is patients with bowel obstructions, which require immediate diversion.
As with all scenarios, however, care plans must be individualized to the particular needs of the patient based on the pattern and pace of metastatic disease, degree of symptoms, risk of imminent obstruction, and comorbidities. For example, patients with a low burden of metastatic disease, a bulky rectal tumor, and a high likelihood of long-term survival may benefit from treatment of the primary tumor to prevent symptoms from progressive or recurrent pelvic disease. Since preoperative CMT followed by resection may be the most effective approach for controlling the rectal primary, these patients may be appropriately treated with this regimen. Alternatively, chemotherapy may be provided upfront, and patients who achieve a favorable response may be treated subsequently with consolidative CMT and surgery to provide local control. On the other hand, patients with high-volume metastases and a small, asymptomatic rectal tumor are likely to die of their systemic disease before the primary tumor causes significant symptoms. In such patients, systemic chemotherapy is usually most appropriate, with local pelvic therapy reserved for palliation, if needed.
Management of Liver Metastases
The liver is the most frequent and often the only site of metastasis in CRC. Complete surgical resection of liver metastases can improve survival to an impressive 40% at 5 years and 25% at 10 years post treatment.[7] Therefore, patients who are candidates for surgery, have resectable liver metastases, and have minimal or resectable extrahepatic disease should be directed to operative treatment. Such patients may undergo either a staged or synchronous resection of the metastases and primary rectal tumor.[8-11] There is no consensus regarding the best sequence; rather, institutional philosophy tends to guide management.
The classic approach is surgical removal of the primary tumor, which is considered to be the nidus of metastatic disease, followed by chemotherapy and a second surgery to remove the liver metastases at a later date. If patients progress while on chemotherapy between the two surgeries, the second surgery may not be performed. This approach may be most appropriate for patients who are symptomatic from their primary tumor. Evidence to support this classic approach suggests that the primary tumor affects the liver to promote angiogenesis and metastasis.[12] A synchronous resection of primary tumor and liver metastases obviates the need for two separate operations, but the more arduous surgery may not be suitable for patients with a poor performance status. A more contemporary approach, commonly referred to as “liver-first,” is initial excision of the liver metastases, which demonstrates the genetic mutations and capacity to metastasize, then later resection of the local tumor. Frequently, the primary rectal disease is locally advanced, warranting neoadjuvant CMT; in select patients with a complete clinical response, close observation may delay or abrogate the need for rectal surgery.[13,14] In addition to resection of the primary tumor and liver metastases, systemic chemotherapy improves disease-free and progression-free survival.[15-17] Administration of chemotherapy before or after hepatectomy results in equivalent disease-free and overall survival.[18]
Unfortunately, 70% to 80% of patients with CRC liver metastases are not candidates for resection at initial presentation. Upfront management of patients with unresectable metastases is chemotherapy. Primarily unresectable liver metastases may become resectable after responding to chemotherapy.[19-21] Portal vein embolization or hepatic arterial infusion with floxuridine/dexamethasone may increase the rates of conversion to resectability and thus improve long-term survival.[22,23] For tumors that remain unresectable, nonsurgical liver-directed therapies have yielded promising results and may be considered. For example, high-dose stereotactic body RT is well tolerated and provides local control rates of ≥ 77% at 1 year.[24-26] Radiofrequency ablation (RFA) yields excellent local control of small (< 3 cm) CRC liver metastases.[27-29] Radioembolization, using
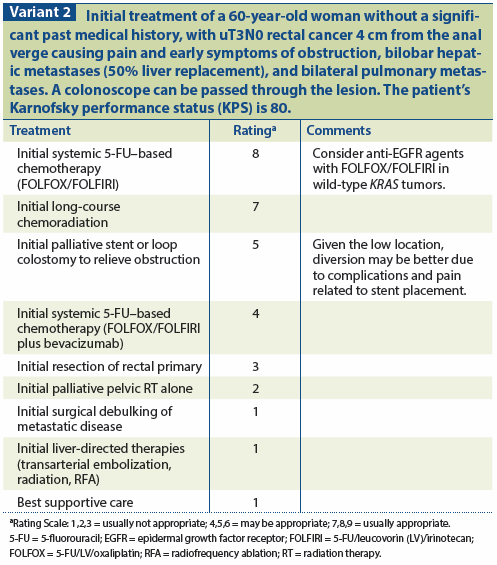
yttrium-90 microspheres in combination with systemic therapy, results in a greater reduction in hepatic metastases than treatment with systemic therapy alone.[30,31] The addition of chemoembolization or cryotherapy to chemotherapy may also improve outcomes and is the topic of ongoing study[32-34] (see Variant 1 and Variant 2).
Cytotoxic and Targeted Therapies
5-FU has been the basis of standard chemotherapy for CRC for the last 5 decades. Continuous-infusion schedules have replaced bolus regimens because they were shown to be more effective and less toxic.[35] Capecitabine, an oral fluoropyrimidine, may be used in place of intravenous 5-FU. Capecitabine is associated with superior response rates and a lower incidence of adverse events, but no significant survival differences are observed when compared to bolus 5-FU/leucovorin (LV).[36] Capecitabine has a dose-limiting toxicity of hand-foot syndrome, which appears to be more common in the US population than in Europe, where most of the studies were conducted. In addition, capecitabine requires a highly motivated and reliable patient who will take oral medication correctly, will not miss or duplicate doses, and will hold medications at appropriate levels of toxicity.
Combining 5-FU/LV or capecitabine with newer agents, including irinotecan and oxaliplatin, has resulted in improved outcomes. Irinotecan, a topoisomerase I inhibitor, can be used independently in 5-FU–resistant advanced CRC or can be combined with 5-FU/LV as first-line therapy in patients with metastatic disease.[37] Oxaliplatin, a third-generation platinum compound, has been shown to be a superior regimen to bolus 5-FU/irinotecan regimens.[38] FOLFOX (5-FU/LV/oxali-platin), FOLFIRI (5-FU/LV/irinotecan), or FOLFOXIRI (5-FU/LV/oxali-platin/irinotecan) are acceptable first-line regimens to treat mCRC in patients appropriate for intensive therapy.[39,40] In patients receiving CMT, addition of oxaliplatin to 5-FU and RT increases toxicity without improving primary tumor response rates, as shown in three randomized controlled trials: STAR (Studio Terapia Adjuvante Retto)-01, ACCORD (Actions Concertées dan les Cancers Colorectaux et Digestifs), and National Surgical Adjuvant Breast and Bowel Project (NSABP) trial R-04.[41] In the metastatic setting, sequential therapy with multiagent chemotherapy before and/or after 5-FU–based CMT is an option to control systemic disease.
New “targeted” therapies such as cetuximab, panitumumab, and bevacizumab have increased the options available for treating metastatic disease. Cetuximab and panitumumab are monoclonal antibodies directed against the epidermal growth factor receptor (EGFR). Cetuximab initially received US Food and Drug Administration (FDA) approval for treatment of irinotecan-resistant disease, in which a 22% response rate was associated with cetuximab/irinotecan therapy vs 11% with cetuximab as a single agent.[42] Panitumumab was FDA-approved after demonstrating improved progression-free survival vs best supportive care in patients with chemotherapy-refractory disease.[43] The discovery that patients with KRAS-mutated tumors do not derive benefit from EGFR-targeted agents has ushered in an era of “personalized” therapy in CRC. For instance, in the large CO.17 study of cetuximab vs best supportive care in chemotherapy-resistant advanced CRC, patients harboring a KRAS mutation had a response rate of 1% and median overall survival time of 4.5 months, whereas those with KRAS wild-type tumors had a response rate of 13% and median overall survival of 9.5 months.[44] In a retrospective meta-analysis of the CRYSTAL and OPUS studies, the addition of cetuximab to chemotherapy resulted in a significant improvement in progression-free and overall survival in patients with KRAS wild-type tumors.[45] Conversely, in a phase III study (COIN) comparing cetuximab in combination with capecitabine or intravenous 5-FU and oxaliplatin vs chemotherapy alone as first-line treatment in mCRC, the former did not meet its primary endpoint of improved overall survival in KRAS wild-type patients (17 months vs 17.9 months; hazard ratio [HR] = 1.04; 95% confidence interval [CI], 0.90–1.20; P = .68).[46] Two recent studies, OPUS[47] and PRIME,[48] demonstrated a progression-free survival benefit with the addition of cetuximab or panitumumab, respectively, to FOLFOX in the first-line setting; however, no benefit was shown for patients with KRAS mutations. These studies collectively suggest that EGFR inhibitors should be considered in treating KRAS wild-type tumors but should not be offered to KRAS-mutant patients. Furthermore, emerging data suggest that patients with KRAS wild-type mCRC receiving FOLFIRI and cetuximab as a first-line treatment experience improved overall survival when compared to those receiving FOLFIRI and bevacizumab.[49]
Bevacizumab is a monoclonal antibody directed against the vascular endothelial growth factor (VEGF). In a randomized phase III trial, adding bevacizumab to bolus 5-FU/LV/irinotecan in patients with advanced CRC improved overall survival by 4.5 months.[50] However, in a larger phase III trial of oxaliplatin-based first-line chemotherapy, the addition of bevacizumab resulted in a modest but significant improvement in progression-free survival but no improvement in response rate and no significant impact on overall survival.[51] In addition, although there were promising initial results with “double biologic” strategies of combining bevacizumab and EGFR-targeting monoclonal antibodies, both the PACCE (panitumumab) and CAIRO2 (cetuximab) trials showed shorter survival times and greater toxicity in the arms with double biologics.[52,53] Thus, bevacizumab should not be combined with other biologic agents but may be used in combination with chemotherapy to treat mCRC. Based on work in animal models, there is concern that administration of an anti-angiogenic agent preoperatively may increase the risk of surgical complications. However, multiple groups have retrospectively shown that surgeries, including liver resections, are safe after bevacizumab delivery.[54,55] Delaying an elective operation until 6–8 weeks (2–3 bevacizumab half-lives) after treatment with bevacizumab is a reasonable consensus practice.
Summary
- Survival of patients with metastatic colorectal cancer (mCRC) has improved significantly in recent years.
- Management of patients with metastatic rectal cancer (mRC) benefits from multidisciplinary input.
- Operative candidates with resectable metastatic disease should undergo resection of the primary tumor and metastases, and should receive chemotherapy.
- Pelvic irradiation with concurrent 5-fluorouracil (5-FU) prior to resection of the rectal tumor is appropriate in patients with bulky, low-lying primary tumors, limited metastatic disease, and a long life expectancy.
- Patients with unresectable metastases should receive upfront chemotherapy.
- Multiple nonsurgical therapies are available to target unresectable liver metastases.
- A combination of cytotoxic and targeted systemic therapies is used in mCRC and has significantly improved outcomes.
- Patients with widespread disease, poor performance status, or multiple comorbidities may be best managed with comfort-oriented, supportive care.
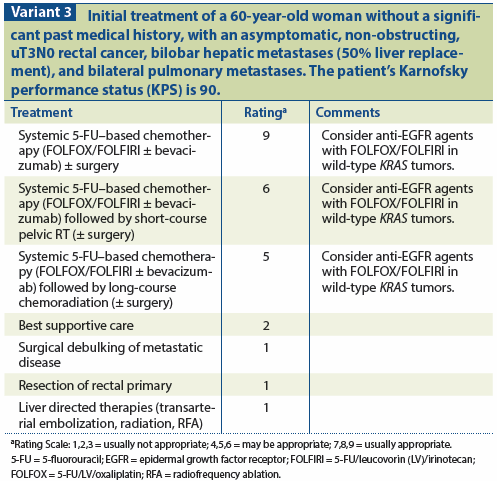
Researchers continue to investigate the role of new targeted therapies in the management of mCRC. Recently, some of these agents have been shown to provide small but statistically significant survival benefits. For example, addition of aflibercept to FOLFIRI resulted in a median overall survival of 13.5 months vs 12.06 months with FOLFIRI and placebo.[56] In a study of patients whose mCRC had progressed on standard therapy, treatment with regorafenib yielded a median overall survival of 6.4 months vs 5.0 months in the placebo group.[57] These and other new targeted agents may play an increasing role in the management of mCRC. Clinical trials should be considered for patients with a good performance status, with the goal of developing more effective therapeutic regimens and rational combinations of chemotherapy, targeted agents, and radiotherapy for patients with mRC (see Variant 3).
Supportive Care
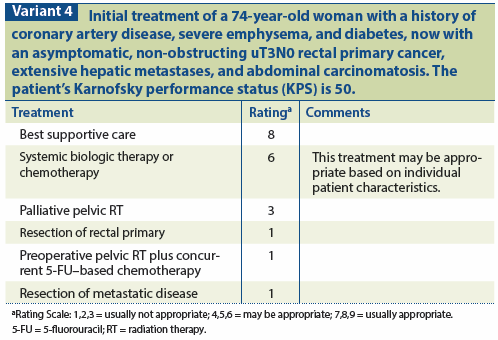
Patients with widespread unresectable mCRC, poor performance status, and multiple comorbidities are often best managed with supportive, comfort-oriented intent. The goals of care should be made clear to these patients, the majority of whom may not understand that their cancer is incurable and treatment is intended to provide palliation only.[58] Local therapies may be valuable for symptomatic relief. For example, palliative RT or CMT achieves at least temporary relief in 80% of patients with mCRC who are suffering from pain, bleeding, or obstruction, with more durable palliation provided by doses ≥ 40 Gy.[59] Stents may also be used to palliate obstruction but may be poorly tolerated in the distal rectum (see Variant 4).
The American College of Radiology seeks and encourages collaboration with other organizations on the development of the ACR Appropriateness Criteria through society representation on expert panels. Participation by representatives from collaborating societies on the expert panel does not necessarily imply individual or society endorsement of the final document.
Financial Disclosure:Joseph M. Herman, MD, reports a conflict of interest related to relationships with Nucletron and Genentech. Prajnan Das, MD, reports research support from Genentech.
Copyright © 2014 American College of Radiology. Reprinted with permission of the American College of Radiology.
For additional information on ACR Appropriateness Criteria®, refer to www.acr.org/ac.
References:
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29.
2. Ries LAG, Young JL Keel GE, et al. SEER survival monograph: cancer survival among adults: US SEER Program, 1988-2001, Patient and tumor characteristics [cancers of the colon and rectum]. 2007. Available from http://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf. Accessed on September 12, 2014.
3. Platell C, Ng S, O'Bichere A, Tebbutt N. Changing management and survival in patients with stage IV colorectal cancer. Dis Colon Rectum. 2011;54:214-9.
4. Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237-48.
5. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40.
6. van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24:1762-9.
7. Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-35.
8. Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934-41.
9. de Jong MC, van Dam RM, Maas M, et al. The liver-first approach for synchronous colorectal liver metastasis: a 5-year single-centre experience. HPB (Oxford). 2011;13:745-52.
10. Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233-41.
11. Tanaka K, Shimada H, Matsuo K, et al. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery. 2004;136:650-9.
12. van der Wal GE, Gouw AS, Kamps JA, et al. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg. 2012;255:86-94.
13. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-7.
14. Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633-40.
15. Ciliberto D, Prati U, Roveda L, et al. Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncol Rep. 2012;27:1849-56.
16. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-16.
17. Sorbye H, Mauer M, Gruenberger T, et al. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg. 2012;255:534-9.
18. Lubezky N, Geva R, Shmueli E, et al. Is there a survival benefit to neoadjuvant versus adjuvant chemotherapy, combined with surgery for resectable colorectal liver metastases? World J Surg. 2009;33:1028-34.
19. Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-57.
20. Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243-9.
21. Masi G, Cupini S, Marcucci L, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol. 2006;13:58-65.
22. Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465-71.
23. Wicherts DA, de Haas RJ, Andreani P, et al. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240-50.
24. Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486-93.
25. Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081-7.
26. Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-8.
27. Kennedy TJ, Cassera MA, Khajanchee YS, et al. Laparoscopic radiofrequency ablation for the management of colorectal liver metastases: 10-year experience. J Surg Oncol. 2013;107:324-8.
28. Siperstein AE, Berber E, Ballem N Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246:559-65.
29. Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958-68.
30. Gulec SA, Pennington K, Wheeler J, et al. Yttrium-90 microsphere-selective internal radiation therapy with chemotherapy (chemo-SIRT) for colorectal cancer liver metastases: an in vivo double-arm-controlled phase II trial. Am J Clin Oncol. 2012;
31. Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687-94.
32. Albert M, Kiefer MV, Sun W, et al. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer. 2011;117:343-52.
33. Ng KM, Chua TC, Saxena A, et al. Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann Surg Oncol. 2012;19:1276-83.
34. Vogl TJ, Gruber T, Balzer JO, et al. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250:281-9.
35. O'Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-7.
36. Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282-92.
37. Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-7.
38. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-47.
39. Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-6.
40. Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-37.
41. Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-44.
42. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-45.
43. Peeters M, Van Custem EV, Siena S, et al. A phase 3, multicenter, randomized controlled trial of panitumumab plus best supportive care (BSC) vs BSC alone in patients with metastatic colorectal cancer [Abstract CP-1]. Presented at: 97th Annual Meeting of the American Association for Cancer Research; April 1–5, 2006; Washington DC.
44. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-65.
45. Van Cutsem E, Rougier P, Köhne C, et al. A meta-analysis of the CRYSTAL and OPUS studies combining cetuximab with chemotherapy (CT) as 1st-line treatment for patients (pts) with metastatic colorectal cancer (mCRC): results according to KRAS and BRAF mutation status (abstr 6.077). Eur J Cancer Suppls. 2009;7:345.
46. Maughan TS, Adams RA, Smith C, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-14.
47. Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-71.
48. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-705.
49. Heinemann V, Fischer von Weikersthal L, Decker T, et al. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS-wildtype metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3). J Clin Oncol. 2013;31:abstr LBA3506.
50. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-42.
51. Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab (Bev) in combination with XELOX or FOLFOX4: Updated efficacy results from XELOX-1/ NO16966, a randomized phase III trial in first-line metastatic colorectal cancer. J Clin Oncol. 2007;25:4028.
52. Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-80.
53. Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-72.
54. D'Angelica M, Kornprat P, Gonen M, et al. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: a matched case-control study. Ann Surg Oncol. 2007;14:759-65.
55. Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173-80.
56. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-506.
57. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-12.
58. Weeks JC, Catalano PJ, Cronin A, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616-25.
59. Bae SH, Park W, Choi DH, et al. Palliative radiotherapy in patients with a symptomatic pelvic mass of metastatic colorectal cancer. Radiat Oncol. 2011;6:52.