Age-Specific Reference Ranges for PSA in the Detection of Prostate Cancer
PSA is the best tumor marker yet discovered. Age-specific reference ranges could improve the sensitivity of PSA by detecting curable, organ-confined tumors.
ABSTRACT: An association between age and prostate-specific antigen (PSA) has been documented: As men age, their serum PSA value increases. Currently, a single demarcation between normal and elevated PSA values, 4.0 ng/mL, is used as an indication for biopsy among men of all ages. The use of age-specific reference ranges might address several shortcomings of the PSA test. First, age-specific reference ranges could improve the sensitivity of PSA by detecting curable, organ-confined tumors in younger men when the threshold of 4.0 ng/mL is lowered. Second, age-specific reference ranges could improve the specificity of the PSA test by raising the PSA threshold for normal among older men. This would modulate PSA interpretation by taking into account the increasing prevalence of both benign prostatic growth and incidental, non-life-threatening cancers among successively older cohorts of men. Furthermore, many unnecessary (false-positive) biopsies would be avoided. However, the association between PSA values and age is not entirely clear, and whether age-specific reference ranges represent the best interpretive index for PSA remains problematic. [ONCOLOGY 11(4):475-485, 1997]
Introduction
Prostate-specific antigen (PSA) is the best "tumor marker" yet discovered.[1,2] This enzyme is produced by the columnar epithelial cells of the prostate and periurethral glands. As a serine protease in the kallikrein gene family, PSA presumably has some biologic function in the prostate or in its secretions.
Semenogelin, a major protein in seminal fluid, is cleaved by PSA, and this cleavage is apparently an important part of the liquefaction of semen.[3] Prostate-specific antigen also cleaves one of the six binding proteins of the insulin-like growth factor, IGF BP-3. In vitro studies have shown that although IGF BP-3 inhibits the activity of IGF-II, its cleavage by PSA reverses this inhibition and frees IGF to stimulate proliferation.[4,5] Unfortunately, far less is known about other biologic substrates of PSA.
The basement membrane of the acini, basal cells lining the acini, and stromal cells act as barriers to prevent the escape of PSA into the bloodstream. Therefore, serum levels of PSA are normally maintained below 4 ng/mL, which corresponds to about 10-6 of the levels of PSA in seminal fluid.[6]
Normal prostate epithelial cells and benign hyperplastic tissue actually produce more PSA protein than does malignant tissue, and PSA messenger RNA (mRNA) is also expressed at higher levels in benign tissue than in malignant prostate tissue.[7,8] Therefore, PSA is not a true tumor marker. Abnormalities in the architecture of the prostate gland resulting from either trauma or disease cause increased "leakage" of the enzyme into the bloodstream via capillaries and lymphatics.[1]
Prostate-specific antigen is specific to the prostate but not to prostate cancer. Prostate cancer can cause a breakdown of the barriers that prevent escape of PSA into the extracellular fluids, resulting in elevated PSA levels in the blood. Prostate-specific antigen levels increase approximately in proportion to the volume of prostate cancer.[9] However, elevated serum levels of PSA do not always result from prostate cancer. Benign conditions, such as bacterial prostatitis, urinary retention, and benign prostatic hyperplasia (BPH), may also cause elevations in serum PSA levels. Although PSA concentrations increase with the volume of BPH tissue, the average increase is small (0.3 ng/mL/g of tissue) when compared with the increase associated with cancer (nearly 3.5 ng/mL/g of malignant tissue).[10]
Early Detection of Prostate Cancer
Prior to the era of PSA testing, the digital rectal examination was the gold standard for the detection of prostate cancer. However, interoperator variance with the digital rectal examination has been documented[11]; its sensitivity is low; and it is more likely to detect locally advanced cancer than organ-confined disease.
The PSA test also is not a flawless method of cancer detection. The major shortcoming of the PSA test is its less-than-perfect sensitivity and specificity rates, despite the fact that these rates are among the highest of cancer screening tests currently in use. A relatively high number of false-positive results is common: PSA levels above normal but resultant negative biopsies.[12] In most screening studies, the positive predictive value of a PSA level 4.0 ng/mL or more is approximately 33%.[13-16]
Combined use of PSA testing and digital rectal examination is universally recommended because of the significant increase in positive predictive value. Approximately 25% to 33% of patients with prostate cancer have serum PSA concentrations in the normal range at the time of diagnosis, made on the basis of a digital rectal examina-tion or a PSA threshold less than 4.0 ng/mL.[15-17] Only 1 patient in 10 with a suspicious digital rectal examination will prove to have prostate cancer on biopsy if the PSA value is less than 4.0 ng/mL.[17,18] When the PSA value is 10.0 ng/mL or higher, at least 50% of patients will have prostate cancer. Between these two groups is the diagnostic "gray zone" of the PSA value, 4.0 to 10.0 ng/mL, for which a positive biopsy of cancer is problematic. In this range, nearly 25% of men will have prostate cancer, and 75% will have benign prostatic enlargement. In fact, approximately 25% of all men with a histopathologic diagnosis of BPH have serum PSA levels higher than commonly used cut-off value of 4.0 ng/mL.[1]
PSA and its Indexes
Various analytic methods have been proposed to improve the sensitivity and specificity of PSA testing. Higher standards are necessary but not sufficient proof that PSA testing will reduce mortality from prostate cancer.[19] These indexes include calculations of PSA density,[20] PSA density adjusted for volume of the transition zone,[21] analysis of PSA velocity,[22] molecular forms of PSA,[23,24] and the application of age-specific PSA reference ranges.[25]
PSA Density
The calculation of PSA density--PSA values in relation to the volume of the prostate gland--was initially proposed by Benson and colleagues[20] as a method to differentiate BPH and cancer. Calculation of PSA density necessarily involves a transrectal ultra- sonographic examination to derive an estimation of volume, and the operator dependency of this examination can influence subsequent calculations of prostatic volume.
Studies of PSA density have reported mixed findings: Some investigators[26,27] have found that PSA density calculation improves the sensitivity and specificity of PSA testing, whereas others[28] have reported no diagnostic improvement with this calculation. Refinements in the interpretation of PSA density calculation have been attempted, such as consideration of the volume of the transition zone[21]; volume-referenced PSA values[29]; and an unpublished study of age-specific PSA density. However, no consistent diagnostic improvement can be claimed for PSA density and its derivatives. A significant correlation between age and volume of the prostate gland has been documented, and this phenomenon supports the consideration of age-specific reference ranges.[25,30]
PSA Velocity
A landmark analysis,[31] based on the Baltimore Longitudinal Study of Aging, demonstrated a significant difference in the age-adjusted rate of change in PSA levels among groups of men who had prostate cancer, BPH, and no prostate disease. Carter and associates[31] suggested that a 0.75-ng/mL/yr increase in PSA is predictive of a diagnosis of cancer. However, intraindividual variation of PSA test results, the lack of standardization of PSA assays, and the recommendation that a minimum of three annual tests over a 2-year period be used to calculate PSA velocity, have hampered the establishment of a "normal" PSA change (slope, or velocity) over time. An association between age-specific reference ranges and PSA velocity seems to be intuitively apparent, but long-term prospective studies of such an association have not yet been reported.
Different Molecular Forms of PSA
Considerable enthusiasm has been generated by the possibility of differentiating benign and malignant disease through the assay of the proportion of different molecular forms of PSA: (1) PSA bound to alpha-1-antichymotrypsin, (2) PSA bound to alpha-2-macro-globulin, and (3) unbound (free) PSA. However, establishing a "correct" ratio of these different PSA types that will distinguish prostate cancer from benign growth will likely be debated, much as PSA and its indexes are debated currently. Although the amounts of these different molecular forms and of total PSA apparently vary according to age, the ratios do not, and may ultimately obviate any age-related consideration in diagnostic evaluations.[32]
Age-Specific PSA Reference Ranges
Studies Establishing Age-Specific Ranges
Early studies of PSA testing for the detection of prostate cancer found consistent, strong associations between PSA values and glandular volume, PSA values and age, and age and glandular volume.[14,30,33] Brawer and colleagues[14] found that the mean PSA value increased with age (P = less than .0001), but the correlation coefficient (r)--the degree of correlation between age and PSA values--was only .15. (It is important to note that when the correlation coefficient is squared [r2], resulting in a coefficient of determination, that percent explains the linear relationship between the two variables. In the case of the findings of Brawer's group,[14] this would mean that 2.3% of the variation in PSA levels can be explained by its linear relationship to age).
Babaian and colleagues[33] also found a significant association between PSA levels and age and a significant relationship between prostatic volume (determined by transrectal ultrasonography) and increasing PSA levels and age (for all, P less than .00005). The increasing prevalence of both benign prostate disease and prostate cancer as men age had long been reported, and PSA seemed to be related to this phenomenon. This study recommended biopsy when the PSA value is between 4.0 to 10.0 ng/mL and the prostatic volume is 25 ccor less.
Collins and associates[30] reported on a clinic population referred for assessment of BPH. The study's objective was to investigate the relationships between prostatic volume and PSA levels and between age and prostatic volume. Correlations were established, but the study also found an independent association between PSA levels and age. Linear regression analysis showed an independent association between PSA levels and age when adjustments were made for prostate volume. Age and prostate volume influence PSA levels independently. A modest correlation of PSA levels and age was reported (r = .37; P less than .0001).
TABLE 1

Age-Specific Reference Ranges for PSA Values
The seminal study of age-specific reference range by Oesterling and associates[25] reported on a community-based population (N = 471) that had no evidence of prostate cancer. Prostate-specific antigen levels correlated with age (r = .43 [r² = 18.5]; P less than .0001); a higher correlation of PSA with PSA density was found (r = .55; P less than .0001); and PSA density was weakly correlated with age (r = .25; P = less than .001). With the median PSA value plus two standard deviations, age-specific reference ranges were established for 10-year age groups. These age-specific reference ranges have become the standard in the literature (Table 1). Based on regression analysis of the cross-sectional data, this study estimated that the serum PSA concentration increases approximately 3.2% per year. For a healthy, 60-year-old man, that would mean an increase of .04 ng/mL over the next year.
Oesterling has been at the forefront of advocating the use of age-specific reference ranges to make PSA a more discriminating tumor marker for detecting clinically significant cancers in older men (increasing specificity by raising the threshold for normal PSA levels) and potentially curable cancers in younger men (increasing sensitivity by lowering the threshold for normal PSA levels).[34,35] Other studies[36,37] have investigated this association between PSA levels and age, and similar age-specific reference ranges have been proposed (Table 1).
The age-specific reference ranges of Oesterling's group[25] are relatively conservative, given the generally lower ranges in each age group. This may reflect less PSA variability among the relatively homogeneous male population of Olmstead County, Minnesota. Their age-specific reference ranges may reflect a standard against which "abnormally" high PSA levels can be evaluated. The incidence of prostate cancer may also be lower in Olmstead County because of the number of research studies conducted among its population. As increasing percentages of men undergo PSA testing, and as more cancers are detected, regression to the mean PSA values of Olmstead County may occur throughout the country.
Application to Clinical Study Results
The age-specific reference ranges established by Oesterling's group[25] have been applied retrospectively to a number of studies and have substantiated the contention that this PSA index increases sensitivity among younger men and specificity among older men. In one study[38] of 2,998 men more than 60 years of age from a single urologic practice, age-specific reference ranges decreased sensitivity by 9% but increased specificity by 11% and positive predictive value by 5%. If these reference ranges had been used, 92 biopsies (5.5%) would have been avoided, and only 19 (.6%) cancers would not have been detected. However, of these 19 cancers, 13 were detected in men ³ 70 years of age and 18 were unlikely to be of clinical consequence, based on organ confinement, tumor volume, and Gleason grade.
Partin and colleagues[39] reviewed the records of 4,597 men with clinically localized prostate cancer from the extensive Johns Hopkins surgical series. Using the standard reference PSA value of 4.0 ng/mL alone, they found that 82% of tumors would have been detected; age-specific reference ranges would have detected 78%. Age-specific reference ranges would have found 18% more cancers in men less than 60 years of age and 22% fewer cancers in men 60 years of age or more.
In men less than 60 years of age, 74 cancers would have been detected by age-specific reference ranges but not by the standard reference. Moreover, of these 74 cancers, 60 (81%) had a favorable pathologic status: either organ confinement or capsular penetration with a Gleason grade of less than 7. In men 60 years of age or more, 252 tumors would not have been detected, but of these tumors, 76% (191) had a favorable pathologic status. This study concluded that age-specific reference ranges are most useful in men 60 years of age or more with T1c prostate cancer (ie, they increase specificity) because 95% of the "missed" tumors among older men had a favorable pathologic status.
Another study, apparently based on results from early detection programs, concurred that age-specific reference ranges increased sensitivity in detecting prostate cancer in younger men, who would be more likely to benefit from treatment, while decreasing the biopsy rate in older patients.[40] These investigators concluded that age-specific reference ranges would be most valuable for patients more than 70 years of age, among whom 22% would have been spared transrectal ultrasonography with biopsy. Similarly, Lankford and colleagues[41] found a significant association between PSA levels and age (r = .33) and a significant improvement in specificity when age-specific reference ranges were applied to men 70 years of age or more , as compared with the standard PSA reference (58.6% and 34.2%, respectively).
Two European studies[42,43] reported results based on the application of age-specific reference ranges to findings from screening populations. Reissigl and associates[42] reported that an 8% increase in biopsies among younger men (in their 40s and 50s) would have occurred with age-specific reference ranges, but this would have effected an 8% increase in the detection of organ-confined cancer. In older men (more than 60 years), 21% fewer biopsies would have been performed and 4% of organ-confined tumors would have been missed. Using age-specific reference ranges and digital rectal examination as indicators for biopsy, Bangma and colleagues[43] reported that a 37% reduction in biopsies would have occurred, with a 12% loss of detected cancers, all of which were nonpalpable and organ-confined .
Although these studies generally support the use of age-specific reference ranges to improve the characteristics of PSA testing, they do not provide sufficient evidence for their universal application. The variability of findings likely reflects the diagnostic capabilities of the clinical investigators, the detection technologies employed, and the different patient populations. One autopsy study confirmed an increased specificity with age-specific reference ranges among men more than 60 years of age,[44] but such studies simply show that cancers are missed and an elevated PSA value (more than 4.0 ng/mL) alone does not indicate cancer.
Criticisms of Age-Specific Ranges
Widespread acceptance of age-specific PSA reference ranges as a valuable index for the effective and efficient detection of prostate cancer has been compromised by negative clinical findings, philosophy, and the continued adequate performance of the standard reference PSA value of 4.0 ng/mL. Age-specific reference ranges have theoretical appeal, but study results are not consistent with regard to the percentage and type of prostate cancers not detected among older men when age-specific reference ranges have been applied retrospectively.
Data from the American Cancer Society's National Prostate Cancer Detection Project showed that age-specific reference ranges increased specificity but at a nonsignificant loss of sensitivity.[45] A PSA value of 4.0 ng/mL alone had a sensitivity of 71.9% and a specificity of 90%; age-specific reference ranges had a sensitivity of 67.3% and a specificity of 90.9%.
A German study concluded that the application of age-specific reference ranges (or any PSA value less than 3.1 ng/mL) does not improve the test's sensitivity among men younger than age 50. Moreover, using a higher PSA value (7.5 ng/mL) for men more than 70 years of age does improve specificity but lowers sensitivity.[46]
Although Oesterling and colleagues[38] and Partin's group[39] each noted that only 5% of cancers undetected with age-specific reference ranges had unfavorable histology, a recent study[47] reported that 60% (9 of 15) of cancers that would have remained undetected by age-specific reference ranges had histologic characteristics qualifying them as large, life-threatening, and clinically significant. All of these cancers were in men younger than age 75.
In another extensive critique of age-specific reference ranges, Catalona and associates[48] concluded that if an age-specific reference of 3.5 ng/mL had been used for men between the ages of 50 and 59, there would have been a 45% increase in biopsies, with only a projected 15% increase in cancer detection. If an age-specific reference of 4.5 ng/mL had been used for men between the ages of 60 and 69, 15% fewer biopsies would have occurred, but 8% of organ-confined tumors would have been missed. Increasing the normal PSA cut-off value to 6.5 ng/mL for men more than 70 years would have resulted in 44% fewer biopsies (70 of 159) and would have missed 47% of detected organ-confined cancers (7 of 15). This study concluded that a PSA value of 4.0 ng/mL need not be altered for older men because the number of biopsies performed for each cancer detected remained constant across age groupings, apparently unaffected by the simultaneously increasing prevalence of BPH and cancer with age.
In light of the fact that both sensitivity and specificity cannot be improved simultaneously, the philosophical issue surrounding age-specific reference ranges is whether sensitivity or specificity should take priority. Advocates of screening and early detection of prostate cancer favor sensitivity over specificity because more clinically important, organ-confined disease will be detected.[49] In fact, it has been proposed that the threshold for normal be lowered from 4.0 to 2.5 ng/mL for everyone.[50] A more provocative proposal has been to ignore PSA values and age-specific reference ranges completely and simply to perform a biopsy on all men at 50 years of age[51]; specificity would be sacrificed at the altar of universal detection.
These and similar proposals may not be implemented because of the relatively satisfactory performance of a single demarcation between normal and elevated PSA levels.[52] The reference for the maximum normal PSA value (4.0 ng/mL) was established with a population mix younger than would be normally tested for prostate cancer: 472 individuals, of whom only 55 were between the ages of 50 and 59 and 19 were 60 years of ageor more.[53] This basis may not reflect the increasing risk of prostate cancer with increasing age, but the objective was to identify a demarcation between normal and elevated PSA levels. Unless another standard becomes more acceptable, the imperfect nature of the PSA test may simply require such a norm; ie, below 4.0 ng/mL to detect more organ-confined cancers regardless of the higher number of false-positive results or above 4.0 ng/mL to detect fewer clinically insignificant cancers while missing more clinically significant cancers.
Variations on the Age-Specific Reference Range Theme
Attempts to complement the value of age-specific reference ranges in the detection of prostate cancer have included consideration of prostatic volume after age-specific reference ranges and proposed volume-referenced PSA values. Meshref and colleagues[54] studied PSA density with age-specific reference ranges. In a clinically referred population (N = 2,429), transrectal ultrasonography was performed on everyone. This experience alone indicated proficiency in establishing comparable estimations of volume and led to the finding that volume is more strongly correlated to PSA value (r = .46) than are PSA values to age (r = .25). Prostate-specific antigen density (which directly relates serum PSA values to prostatic volume), however, showed a weak correlation with age (r = .1).
In multiple regression analysis, prostate volume accounted for 18% of the variation in serum PSA values, whereas age accounted for only an additional 2%. In the patient group with PSA values between the age-specific upper limit of normal and 10.0 ng/mL, the positive rate of biopsy in patients with a PSA density less than .15 was only 1.9% (2/108/315), but in patients with a PSA density more than .15, the positive rate of biopsy was 27.2% (59/217/240). This study concluded: (1) that age-specific reference ranges do not totally account for the effect of prostate volume on serum PSA values and (2) that PSA density can still safely be used to reduce the number of biopsies performed in patients with a normal digital rectal examination and transrectal ultrasonography, as well as a serum PSA level 10.0 ng/mL or less but above the age-specific reference range limit of normal.
Babaian and associates[29] also attempted to address the mixed results of studies of PSA density and age-specific reference ranges. Because the association of glandular volume with PSA values is stronger than that with age, these investigators developed the concept of volume-referenced PSA testing and compared its sensitivity and specificity with other indices.
Volume-referenced PSA testing was more sensitive but less specific than age-specific reference ranges and PSA density; it was only equivalent to PSA testing clinically. Age-specific reference ranges would have prevented more biopsies than volume-referenced testing (39% vs 31%) but would have resulted in the diagnosis of 48% fewer cancers. Receiver-operator characteristic curve analysis demonstrated a better performance for volume-referenced PSA testing and PSA density than for PSA testing or age-specific reference ranges.
In the PSA value range of 4 to 10 ng/mL, volume-referenced testing was superior to PSA testing (P = less than .01) and age-specific reference ranges (P less than .001) and equivalent to PSA density. In this range, age-specific reference ranges missed the fewest cancers (6%) but resulted in the fewest biopsies (12%). However, a key point of this study was that enhanced detection of prostate cancer was inversely associated with increasing volume of the prostate gland and directly related to increasing patient age.[29]
The relationship between age-specific reference ranges and PSA change--PSA velocity--offers another potential PSA index that could improve the test's characteristics. Pearson and colleagues[55] studied annual PSA velocity rates in 727 men (age range, 35 to 92 years) with no history of prostate cancer and a PSA level < 10 ng/mL. The mean PSA velocity and the upper limits of PSA velocity increased significantly with age (P less than .001). Age-specific values for PSA velocity were suggested: less than 50 years of age, 0.125 ng/mL/yr; 50 to 59 years old, 0.15 ng/mL/yr; 60 to 69 years old, 0.3 ng/mL/yr; and 70 years older more, 0.4 ng/mL/yr.[55]
The shortcoming of this study, however, is the particular PSA range of values selected (ie, 4.0 to 10.0 ng/mL) to establish age-specific PSA velocity: Whatever range of PSA values is used to establish "normal" PSA velocity will directly influence the resultant PSA velocity determinations. If, for example, a PSA value of 0.0 to 4.0 ng/mL is used to establish PSA velocity levels, the age-specific rates will be much lower. Again, it becomes a question of whether to emphasize sensitivity or specificity in the detection of prostate cancer by using age-specific PSA velocity values.
How Best to Use the PSA-Age Relationship
TABLE 2

Mean PSA Values by Age
As discussed elsewhere,[37] age-specific reference ranges are a result of the increasing mean PSA values and the increasing PSA variance (standard deviation) in successively older cohorts of men. Mean PSA values are related to the population studied and may also be related to the time during which a particular study was conducted (Table 2). The variability in PSA values as men age suggests that caution should be exercised when considering the clinical implications of age-specific reference ranges.[56] The upper limits are established by two methods: (1) extending mean PSA levels upward two standard deviations to establish a 95% confidence limit, or (2) extending the median PSA value to the 95th percentile. However, the distribution of PSA values in any age group is not a normal distribution: absolute 0.0 ng/mL is always the lowest value.
TABLE 3

Median PSA Values and Interquartile Ranks
The degree of variation in PSA values increases with each older age cohort. That is, men in their 70s exhibit greater variability in PSA values than men in their 60s, who, in turn, exhibit greater variability than men in their 50s, with men in their 40s demonstrating the least variability. In fact, Bangma and associates[43] have shown that the coefficient of correlation weakens as older ages are considered: r = .32 for all men but only .16 for men 50 years or more. Table 3 presents the interquartile ranges for 10-year age cohorts, and the increasing variance on either side of the median--especially between the 50th and 75th percentile--is apparent.
FIGURE 1
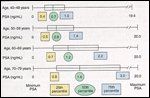
Variability in PSA With Age
If the variance in PSA values is related to the "normal" pattern of prostatic function, it would stand to reason that men in their 40s, when benign prostatic enlargement and prostate cancer are uncommon, would show the least variance in PSA values. With increasing age and greater variability in circulating androgen levels, various endogenous prostatic insults, and other physiologic factors that may be environmentally influenced, some prostates will be stimulated to grow--whether that growth is benign or malignant--whereas others will not.[57] Thus, greater variability is found among PSA levels of successively older men (Figure 1).
Age-specific reference ranges will necessarily be less sensitive than the standard of 4.0 ng/ml among older men for two major reasons: (1) poorly differentiated cancers in small-volume prostates will not produce ("leak") sufficient PSA to cross even the current threshold of 4.0 ng/mL let alone a higher threshold; and (2) the variance in PSA values is greater among older men. The men with large-volume prostates bias the age-specific reference ranges for men with normal-volume prostates in every age group.
TABLE 4

Age-Specific Reference Ranges for PSA Values: 1-Year, 5-Year, and 10-Year Calculations
Ten-year age groupings for age-specific reference ranges may simply be too inclusive (and less sensitive than 4.0 ng/mL) for clinical applicability at this time. Five-year age-specific reference ranges, or even an annual reference point (at the 95th percentile), may provide a sound criterion for clinical interpretation. Based on more than 77,000 individual PSA test results, previously analyzed in 10-year age groups,[37] Table 4 presents the increasingly more specific reference points by 10-, 5-, and 1-year age groupings. For each year of age after the age of 45, more than 1,000 PSA test results provided the basis for computation.
Albeit subject to the same shortcomings of 10-year age-specific reference ranges, an annual reference point may help interpret PSA test result.
Conclusions
Oesterling[25,34,35] has eloquently stated the potential advantages of using age-adjusted PSA standards: (1) to increase the test's sensitivity among younger men, detecting more curable, organ-confined tumors earlier; and (2) to increase the test's specificity among older men, avoiding many unnecessary biopsies. Surely, determining the most effective way of using PSA testing to decide whether or not to perform a biopsy is a complex problem. Attempts to find an easy solution to this problem should be tempered by the dictum of H. L. Mencken: "For every complex problem there is a simple solution, and it is wrong."
The issue of age-specific reference ranges has emerged because of the lack of sufficient evidence that screening and early detection of prostate cancer with PSA testing improves survival. If the objective is to find curable organ-confined prostate cancers, notwithstanding the debate over labels of "significant" or "insignificant" tumors, sensitivity must be the prime criterion; ie, one needs to perform more biopsies so as not to miss any clinically significant, curable, organ-confined tumors. Studies have shown that the majority of PSA-detected cancers are significant.
If, on the other hand, the objectives are to increase specificity, to attempt to limit cost-effectively the number of biopsies performed in older men[35] and to minimize the psychological consequences of false-positive results, age-specific reference ranges make sense. Some curable cancers would be missed in older men with this approach, however.
The assumption here is that with the increasing incidence of cancer as men age, one standard cut-off value for PSA (4.0 ng/mL) would tip the scales toward detecting more insignificant tumors. The trade-off between sensitivity and specificity should be the clue that the test is imperfect as it stands. Although there is no easy answer, studies of the molecular forms of PSA and the ratio of free-to- total PSA may render this debate moot by permitting a pre-biopsy determination of benign or malignant disease. However, the single best ratio of free-total PSA will likely be as controversial as any current PSA index.
References:
1. Oesterling JE: Prostate specific antigen: A critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol 145:907-923, 1991.
2. Partin AW, Oesterling JE: The clinical usefulness of prostate specific antigen: Update 1994. J Urol 152:1358-1368, 1994.
3. Lilja H: A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest 76:1899-1903, 1985.
4. Cohen P, Peehl DM, Lamson G, et al: Insulin-like growth factors (IGFs), IGF receptors, and IGF binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab 73:401-407, 1991.
5. Cohen P, Graves HCB, Peehl DM, et al: Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab 75:1046-1053, 1992.
6. Christensson A, Laurell C-B, Lilja H: Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem 194:755-763, 1990.
7. Wang MC, Valenzuela LA, Murphy GP, et al: Purification of human prostate-specific antigen. Invest Urol 17:159-163, 1979.
8. Qui S-D, Young CY, Bilhartz DL, et al: In situ hybridization of prostate-specific antigen mRNA in human prostate. J Urol 144:1550-1556, 1990.
9. Schmid H-P, McNeal JE, Stamey TA: Observations on the doubling time of prostate cancer. Cancer 71:2031-2040, 1993.
10. Stamey TA, Yang N, Hay AR, et al: Prostate specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317:909-916, 1987.
11. Smith DS, Catalona WJ: Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 45:70-75, 1995.
12. Littrup PJ, Kane RA, Mettlin CJ, et al: Cost-effective prostate cancer detection: Reduction of low-yield biopsies: The investigators of the American Cancer Society National Prostate Cancer Detection Project. Cancer 74:3077-3079, 1994.
13. Catalona WJ, Smith DS, Ratliff TL, et al: Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 324:1156-1161, 1991.
14. Brawer MK, Chetner MP, Beatie J, et al: Screening for prostatic carcinoma with prostate specific antigen. J Urol 147:841-845, 1992.
15. Labrie F, Dupont A, Suburu R, et al: Serum prostate specific antigen: A prescreening test for prostate cancer. J Urol 147:846-852, 1992.
16. Catalona WJ, Richie JP, Ahmann FR, et al: Comparison of digital rectal examination and serum prostate specific antigen in early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J Urol 151:1283-1290, 1994.
17. Hudson MA, Bahnson RR, Catalona WJ: Clinical use of prostate specific antigen in patients with prostate cancer. J Urol 142:1011-1017, 1989.
18. Richie JP, Catalona WJ, Ahmann FR, et al: Effect of patient age on early detection of prostate cancer with serum prostate-specific antigen and digital rectal examination. Urology 42:365-374, 1993.
19. Begg CB: Methodological issues in studies of the treatment, diagnosis, and etiology of prostate cancer. Semin Oncol 21:569-579, 1994.
20. Benson MC, Whang IS, Olsson CA, et al: The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol 147:817-821, 1992.
21. Kalish J, Cooner WH, Graham Jr SD: Serum PSA adjusted for volume of transition zone (PSAT) is more accurate than PSA adjusted for total gland volume (PSAD) in detecting adenocarcinoma of the prostate. Urology 43:601-606, 1994.
22. Carter HB, Pearson JD: PSA velocity for the diagnosis of early prostate cancer: A new concept. Urol Clin North Am 20:665-670, 1993.
23. Lilja H: Significance of different molecular forms of serum PSA: The free, noncomplexed form of PSA versus that complexed to a1-antichymotrypsin. Urol Clin North Am 20:681-686, 1993.
24. Catalona WJ, Smith DS, Wolfert RL, et al: Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA 274:1214-1220, 1995.
25. Oesterling JE, Jacobsen SJ, Chute CG, et al: Serum prostate-specific antigen in a community-based population of healthy men: Establishment of age-specific reference ranges. JAMA 270:860-864, 1993.
26. Seaman E, Whang M, Olsson CA, et al: PSA density (PSAD): Role in patient evaluation and management. Urol Clin North Am 20:653-663, 1993.
27. Jewett MAS, Jain U, Toi A, et al: Prostate specific antigen density (PSAD): Can it distinguish between benign hyperplasia and malignancy (abstract)? J Urol 149:415A, 1993.
28. Brawer MK, Aramburu FAG, Chen GL, et al: The inability of prostate specific antigen index to enhance the predictive value of prostate specific antigen in the diagnosis of prostatic carcinoma. J Urol 150:369-373, 1993.
29. Babaian RJ, Kojima M, Ramierez EI, et al: Comparative analysis of prostate specific antigen and its indexes in the detection of prostate cancer. J Urol 156:432-437, 1996.
30. Collins GN, Lee RJ, McKelvie GB, et al: Relationship between prostate specific antigen, prostate volume, and age in the benign prostate. Br J Urol 71:445-450, 1993.
31. Carter HB, Pearson JD, Metter EJ, et al: Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA 267:2215-2220, 1992.
32. Oesterling JE, Jacobsen SJ, Klee GG, et al: Free, complexed, and total serum prostate specific antigen: The establishment of appropriate reference ranges for the concentrations and ratios. J Urol 154:1090-1095, 1995.
33. Babaian RJ, Miyashita H, Evans RB, et al: The distribution of prostate specific antigen in men without clinical or pathological evidence of prostate cancer: Relationship to gland volume and age. J Urol 147:837-840, 1992.
34. Oesterling JE: The importance of patient age and race in establishing appropriate reference ranges for serum PSA. Proceedings from the 4th International Symposium on Recent Advances in Urological Cancer Diagnosis and Treatment. Paris, France, June 22-24, 1994.
35. Oesterling JE: Using prostate-specific antigen to eliminate unnecessary diagnostic tests: Significant worldwide economic implications. Urology 46(suppl 3A):26-33, 1995.
36. Dalkin BL, Ahmann FR, Kopp JB: Prostate specific antigen levels in men older than 50 years without clinical evidence of prostatic carcinoma. J Urol 150:1837-1839, 1993.
37. DeAntoni EP, Crawford ED, Oesterling JE, et al: Age- and race-specific reference ranges for prostate-specific antigen from a large community-based study. Urology 48:234-239, 1996.
38. Oesterling JE, Jacobsen SJ, Cooner WH: The use of age-specific reference ranges for serum prostate specific antigen in men 60 years old or older. J Urol 153:1160-1163, 1995.
39. Partin AW, Criley SR, Subong ENP, et al: Standard versus age-specific prostate specific antigen reference ranges among men with clinically localized prostate cancer: A pathological analysis. J Urol 155:1336-1339, 1996.
40. El-Galley RES, Petros JA, Saunders WH, et al: Normal range prostate-specific antigen versus age-specific prostate-specific antigen in screening prostate adenocarcinoma. Urology 46:200-204, 1995.
41. Lankford SP, Peters KL, Elser RC: Potential effects of age-specific reference ranges for serum prostate-specific antigen. Eur Urol 27:182-186, 1995.
42. Reissigl A, Pointer J, Horninger W, et al: Comparison of different prostate-specific antigen cutpoints for early detection of prostate cancer: Results of a large screening study. Urology 46:662-665, 1995.
43. Bangma CH, Kranze R, Blijenberg BG, et al: The value of screening tests in the detection of prostate cancer. Part II: Retrospective analysis of free/total prostate-specific analysis ratio, age-specific reference ranges, and PSA density. Urology 46:779-784, 1995.
44. Speights Jr VO, Brawn PN, Foster DM, et al: Evaluation of age-specific normal ranges for prostate-specific antigen. Urology 45:454-458, 1995.
45. Mettlin C, Littrup PJ, Kane RA, et al: Relative sensitivity and specificity of serum prostate specific antigen (PSA) level compared with age-referenced PSA, PSA density, and PSA change. Cancer 74:1615-1620, 1994.
46. Weichert-Jacobsen K, Tilmann L: Clinical significance of prostate specific antigen age-specific reference ranges. J Urol 153:465A, 1995. Abstract.
47. Borer JG, Sherman J, Solomon MC, et al: Age-specific reference ranges for prostate specific antigen and digital rectal examination may not safely eliminate further diagnostic procedures. J Urol 155:322A, 1996. Abstract.
48. Catalona WJ, Hudson MA, Scardino PT, et al: Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: Receiver operating characteristic curves. J Urol 152:2037-2042, 1994.
49. Catalona WJ, Smith DS: Comparison of different serum prostate specific antigen measures for early prostate cancer detection (editorial). Cancer 74:1516-1518, 1994.
50. Catalona WJ: What to do when the PSA is 'abnormal.' Proceedings from the American Urological Association Meeting, Orlando, Florida, May 6, 1996.
51. Fleming C, Barry MJ, Oesterling JE: Prostate-specific antigen (PSA) age-specific reference ranges versus a single threshold: Is the best answer to bypass PSA and biopsy everyone (abstract)? J Urol 155:452A, 1996.
52. Petteway J, Brawer MK: Age specific vs 4.0 ng/mL as a PSA cutoff in the screening population: Impact on cancer detection (abstract). J Urol 153:465A, 1995.
53. Myrtle JF, Klimley PG, Ivor LP, et al: Clinical utility of prostate specific antigen (PSA) in the management of prostate cancer, in Advances in Cancer Diagnostics. San Diego, Hybritech, Inc., 1986.
54. Meshref AW, Bazinet M, Trudel C, et al: Role of prostate-specific antigen density after applying age-specific prostate-specific antigen reference ranges. Urology 45:972-979, 1995.
55. Pearson JD, Carter HB, Metter EJ, et al: Sensitivity and specificity of age-specific reference ranges for PSA velocity (abstract). J Urol 153:465A, 1995.
56. Anderson JR, Strickland D, Corbin D, et al: Age-specific reference ranges for serum prostate-specific antigen. Urology 46:54-57, 1995.
57. Nadler RB, Humphrey PA, Smith DS, et al: Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol 154:407-413, 1995.