Anaphylaxis: Implications of Monoclonal Antibody Use in Oncology
The phenomenon of anaphylaxis was discovered by Portier and Richet in 1903.[1,2] They injected dogs with toxins from sea anemone with the intent of generating protective antibodies. Unexpectedly, they found that certain dogs became ill with a rapid heartbeat and collapse. Because this syndrome was the precise opposite of protection or prophylaxis, they termed it anaphylaxis.
Anaphylaxis is currently classified as an immunologically triggered response with reactions that are IgE-mediated and reactions that are not IgE-mediated. This immunologically mediated phenomenon can result in various clinical manifestations, including decreased blood pressure, generalized skin infl ammation, such as hives and pruritus, and respiratory symptoms, such as wheezing or bronchospasm. The severity of anaphylaxis can range from a mild allergic reaction to a potentially fatal anaphylactic shock. Numerous causative agents trigger anaphylactic reactions, and some of the best described include food and bee sting allergens. Monoclonal antibodies, which are increasingly used in the treatment of various malignances, also can cause anaphylaxis. In this review, the mechanisms governing anaphylaxis along with treatment strategies are reviewed. Diagnostic aids for anaphylaxis are also discussed. Increased awareness of the mechanisms, symptoms, and treatment of anaphylaxis can aid caregivers to make informed decisions when new agents, such as monoclonal antibodies, are introduced into the clinic.
The phenomenon of anaphylaxis was discovered by Portier and Richet in 1903.[1,2] They injected dogs with toxins from sea anemone with the intent of generating protective antibodies. Unexpectedly, they found that certain dogs became ill with a rapid heartbeat and collapse. Because this syndrome was the precise opposite of protection or prophylaxis, they termed it anaphylaxis.
Subsequent investigations have shown that anaphylaxis is an immunologically mediated event, most commonly associated with production of IgE antibodies in humans.[3] Animal models have shown that both IgE and IgG antibody classes can trigger anaphylaxis. Mast cells and basophils possess the high affinity IgE receptor, FcεR1, and release mediators of anaphylaxis, including histamine, tryptase, carboxypeptidase A, prostaglandin D2, leukotrienes, and platelet-activating factor (PAF) following activation.[4]
Anaphylaxis is a serious and impressive immunologic reaction with an abrupt, and often unexpected, onset and potentially rapid, fatal outcome if untreated.
Characteristics of Anaphylaxis
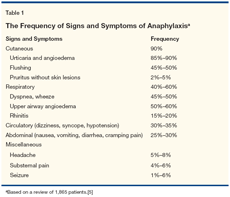
A review of 1,865 patients compiled the approximate frequency of the symptoms and signs characteristic of anaphylaxis (Table 1).[5] Cutaneous findings were most frequent, and 90% of patients undergoing an anaphylactic reaction showed skin changes such as urticaria, angioedema, flushing, and pruritus. Circulatory symptoms, such as dizziness, syncope, and hypotension, were experienced by 30% to 35% of patients, and these were clearly the most alarming, since they signaled impending circulatory collapse.
Diagnosis of Anaphylaxis

An expert panel of clinicians and investigators concerned with immediate hypersensitivity reactions proposed criteria for the diagnosis of anaphylaxis (Table 2).[6] These criteria include an acute onset of illness, within minutes to hours, associated skin or mucosal involvement with hives, pruritus, or fl ushing, and either respiratory symptoms, such as dyspnea, wheezing, or bronchospasm, or a circulatory component with reduced blood pressure. Specifically, a systolic blood pressure of 90 mm Hg or less, or a 30% decrease in systolic blood pressure, qualifies as a criterion for the diagnosis of anaphylaxis.
Criteria for Grading the Severity of Anaphylaxis
Recently, criteria for grading the severity of anaphylaxis have also been utilized.[7,8] These criteria specify three categories:
• Grade 1: acute allergic reaction
• Grade 2: mild to moderate anaphylaxis
• Grade 3: severe anaphylaxis
Patients with an acute allergic reaction (grade 1 anaphylaxis) only have cutaneous and upper respiratory tract findings, such as generalized skin lesions, pruritus, rhinitis/conjunctivitis, urticaria, local edema, and angioedema, without any systemic symptoms or signs or evidence of other organ involvement.
Patients with mild to moderate anaphylaxis (grade 2) have evidence of grade 1 anaphylaxis and respiratory, cardiovascular, gastrointestinal, or neurologic features. The additional features include shortness of breath or dyspnea, wheeze, hoarseness, and nausea or vomiting with associated physical findings, such as bronchospasm, but with systolic blood pressure greater than 90 mm Hg, respiratory rate less than 25/min, and a normal Glasgow Coma Scale score.
Patients with severe anaphylaxis (grade 3) present with the findings listed for grade 2 and potentially life-threatening symptoms or signs. These include one or more of the following:
•Loss of consciousness, syncope, dizziness, or lightheadedness at any time
• Systolic blood pressure less than 90 mm Hg, Glasgow Coma Scale score less than 15 (related to cardiovascular system collapse and/or neurologic dysfunction from hypoperfusion, hypoxia)
•Dyspnea, wheeze, hoarseness, or bronchospasm
•Stridor, cyanosis, laryngeal edema, or a respiratory rate ≥ 25/min
Manifestations of anaphylaxis involving the cutaneous, gastrointestinal, and respiratory organs tend to worsen with each grade and have implications for treatment and life support. Thus, grade 1 anaphylaxis is characterized by involvement of only the skin, mucous membranes, and/or upper airway, whereas grade 2 or mild to moderate anaphylaxis is characterized by the same findings as grade 1 plus involvement of another organ system. Grade 3 or severe anaphylaxis is defined as the same features for grade 2 plus severe life-threatening respiratory or cardiovascular signs.
Classification and Causes of Anaphylaxis
Previously, anaphylaxis had been separated into categories such as true anaphylaxis and anaphylactoid reactions.[ 6] Anaphylactoid reactions were defined as similar to those of anaphylaxis, but triggered by non-IgE mechanisms, such as reactions to nonsteroidal anti-infl ammatory drugs, including aspirin and ibuprofen, reactions to radiocontrast dye exposure, and exercise. More recently, anaphylaxis has been classified as immunologically mediated with subcategories of those reactions that are IgE-mediated and those that are not.[6]
Various agents stimulate IgE antibodies and cause IgE-mediated anaphylaxis. These include exposures primarily through ingestion, injection, or sting. Foods are common anaphylaxis-inducing agents, including peanuts, tree nuts, shellfish, milk, eggs, fish, soy, sesame seeds, and wheat in children, and shellfish in adults.[3] Approximately 3% of young children are allergic to milk[9] and approximately 2% of adults are sensitive to shellfish.[10]
Virtually any oral or injection medication may provoke an anaphylactic reaction, including injections of allergen employed in immunotherapy for the treatment of allergic respiratory disease.[11] An example of anaphylaxis mediated by IgG antibodies are transfusion reactions in patients deficient in IgA who have IgG antibodies to IgA.[12] Although the mechanism of anaphylaxis in IgA-deficient patients has not been thoroughly studied, it seems most likely that formation of immune complexes with fixation of complement and generation of anaphylatoxin are important.[13]
Mechanisms of Anaphylaxis and Illustration of Severity
Anaphylaxis may occur as an abrupt event during the course of other therapies; it has been examined systematically in only a few human studies. Two of these were investigations of venom immunotherapy-one conducted at Johns Hopkins University on the physiologic manifestations of anaphylaxis to stinging insects,[14] and the other in Australia on ant venom anaphylaxis.[15]
effectiveness of venom immunotherapy for the treatment of systemic allergic reactions to insect stings as judged by protection from a deliberate sting.[16] Patients in this study were divided into three categories: one group was treated with placebo, the second with immunization using whole insect body extracts, and a third with insect venom. At the end of the treatment protocol, all patients were subjected to an insect sting. The patients treated with venom were protected against allergic reactions after sting challenge, and this therapy was remarkably superior to placebo and whole-body extract treatment. In this study, 7 of 12 placebo patients and 7 of 11 whole-body extract–treated patients experienced systemic allergic reactions whereas only 1 of 18 patients immunized with venom experienced an acute allergic reaction.[16] Reactions after sting challenge in the 14 patients from the placebo and whole insect body extract–treated groups included urticaria in 11 patients and systemic anaphylaxis in 3 patients.
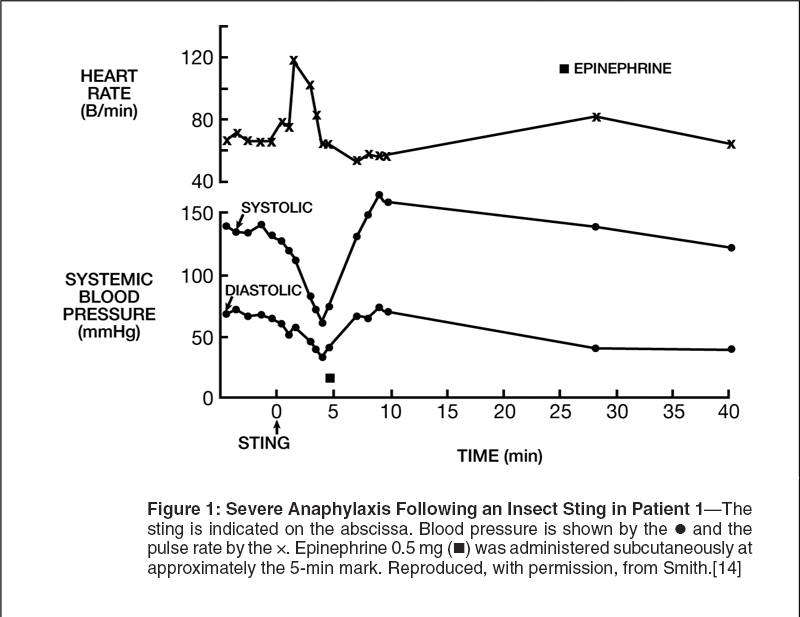
The courses of the reactions in two of the three patients with severe anaphylaxis from the Johns Hopkins immunotherapy study are shown in Figures 1 and 2.[14] Following the sting, patient 1 (Figure 1) complained of dizziness and nausea, and his systolic blood pressure sharply decreased from a baseline value of 140 mm Hg to approximately 60 mm Hg at 4 minutes. Placing the patient in the Trendelenburg position did not affect his blood pressure, but subcutaneous administration of epinephrine was associated with a return to baseline blood pressure during the next 2 to 3 minutes.
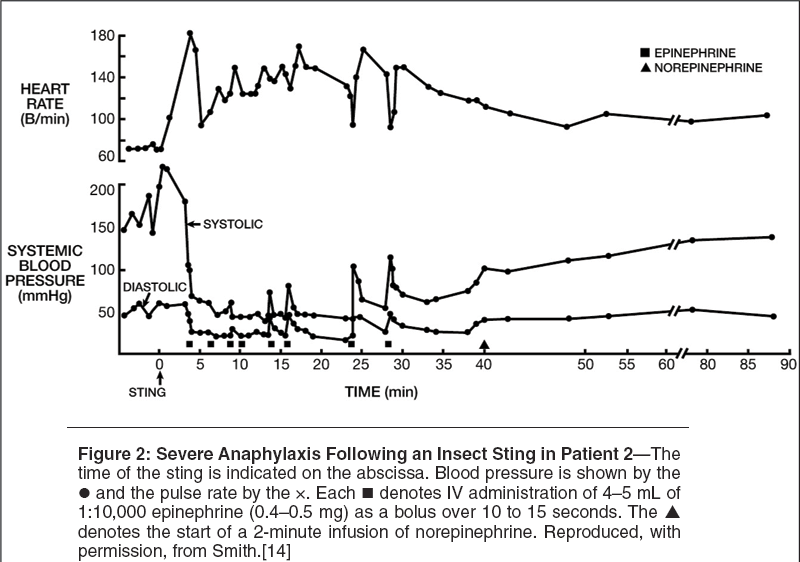
Figure 2 shows the course of anaphylaxis in a second, more severely affl icted patient.[14] No cutaneous manifestations were observed. Treatment was begun with a rapid infusion of physiologic saline and plasma protein fraction (human) 5%, USP (Plasmanate) immediately followed by intravenous administration of 0.5 mg epinephrine. Intravenous treatment with one unit of Plasmanate, 1 liter of saline, and a total of 2 mg of epinephrine did not effect improvement in the mean arterial pressure or pulse pressure during the initial 24 minutes. Subsequently, brief 1- to 2-minute rises in blood pressure occurred, associated with bolus administration of intravenous epinephrine. At 38 minutes, the mean arterial pressure was still 37 mm Hg, and a 2-minute infusion of norepinephrine was administered followed by a rise in blood pressure to 100/45 mm Hg. Thereafter, the arterial pressure continued to rise, but neither it nor the pulse rate had returned to baseline levels during the first 90 minutes of anaphylaxis.
Although the patient denied chest pain, a 12-lead electrocardiogram revealed ST segment depression in leads 2, 3, and aVF. Frequent episodes of vomiting occurred during shock, usually associated with epinephrine injections. Although no obvious respiratory symptoms, such as tachypnea or wheezing, were observed, the PaO2 fell to 52 mm Hg during the anaphylactic reaction and only returned to 86 mm Hg 2 hours after the onset of anaphylaxis and its treatment. The total fl uid and epinephrine therapy administered consisted of 875 mL of Plasmanate (100 mL of Plasmanate contains 5 g of plasma protein, 88% albumin), 1.5 liters of normal saline, and 3.5 mg of epinephrine.
Patients 2 and 3 showed evidence of intravascular coagulation with decreases of factor V, factor VIII, and fibrinogen. These findings indicate that the clotting system is activated during severe anaphylaxis and have implications for the treatment of prolonged anaphylactic reactions.
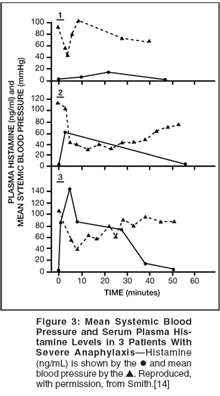
Measurements of plasma histamine during anaphylaxis are shown in Figure 3.[14] The plasma histamine levels in normal individuals were less than 2 ng/mL, whereas in patients experiencing any insect sting with no symptoms or with only urticarial reactions, plasma histamine levels were 2 to 3 ng/mL. Figure 3 shows that plasma histamine levels were dramatically increased in the three patients experiencing severe anaphylaxis and appeared to correlate with the severity of the anaphylactic reaction.
For example, patient 1, with the mildest of the reactions, had a plasma histamine level of approximately 15 ng/mL, whereas patient 3 with the most severe anaphylactic reaction had a level of approximately 140 ng/mL. Note further that the levels of histamine in the patient with the most severe anaphylaxis were still approximately 80 ng/mL at 30 minutes and remained increased for at least 40 minutes.[14]
The Johns Hopkins study of severe anaphylaxis was conducted on a relatively small number of patients and without a defined clinical management protocol. The Australian study on ant sting anaphylaxis analyzed venom immunotherapy in patients with a history of systemic reactions to Myrmecia pilosula, the jack jumper ant responsible for 90% of ant venom anaphylaxis in southeastern Australia.[17] This study showed that venom immunotherapy is highly effective for the prevention of ant venom anaphylaxis. Allergic patients receiving venom immunotherapy were protected from reactions following a deliberate sting. None of the 23 patients had reactions. In contrast, 21 of 29 patients receiving placebo experienced reactions of varying severity but not hypotension, and 8 reactions were associated with hypotension, and thus severe anaphylaxis.
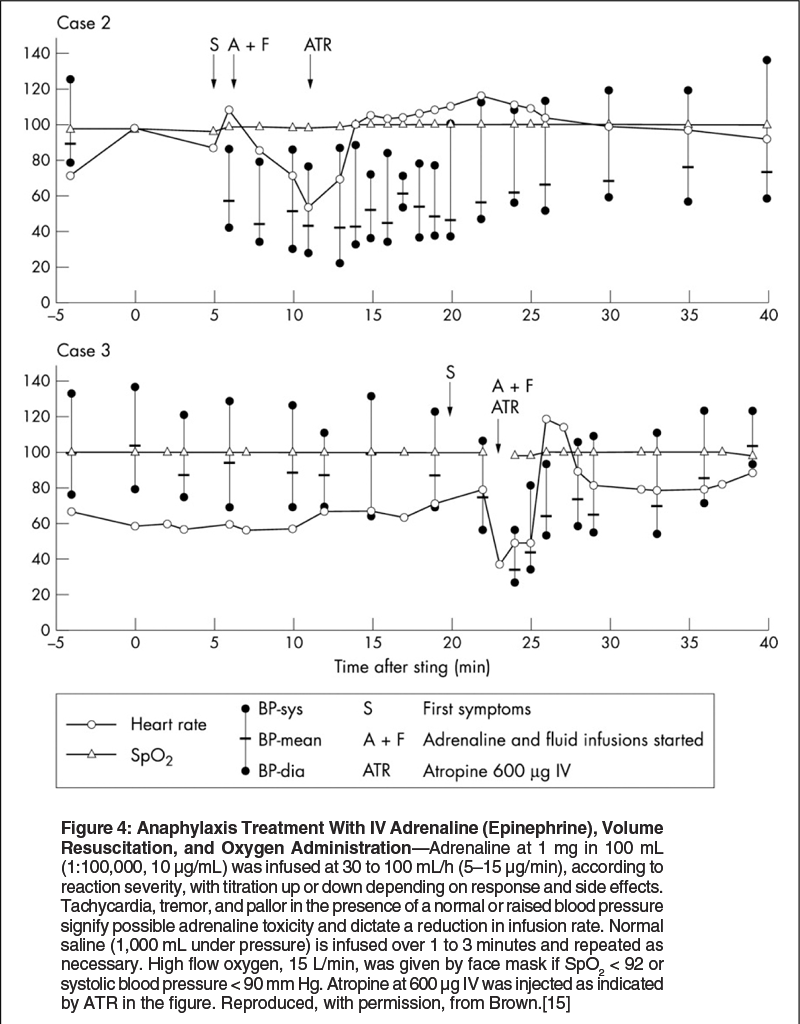
This study prospectively evaluated treatment of anaphylaxis with intravenous adrenaline (epinephrine) and volume resuscitation.[15] Figure 4 shows the course and treatment of anaphylaxis in two patients. Patient 2 developed sudden visual loss, severe headache, and hypotension about 4 minutes after the sting challenge. Despite rapid infusion of 2 liters of normal saline over 5 minutes and adrenaline infusion (10 μg/mL, 5–15 μg/min), progressive bradycardia occurred and was treated with atropine 600 μg intravenously. The patient gradually improved over the following 5 to 10 minutes (Figure 4).
Patient 3 complained of a ‘‘lump in the throat’’ about 20 minutes after the sting challenge, followed 3 minutes later by unconsciousness, agonal respirations, and absent pulses. Adrenaline and saline infusions and atropine effectively restored normal vital signs. One hour after initiation of treatment, the adrenaline infusion was stopped; however, fl orid erythema developed with a fall in blood pressure and therapy was resumed. The patient subsequently recovered uneventfully.[15]
Treatment of Anaphylaxis
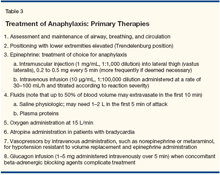
The essentials of anaphylaxis treatment have recently been reviewed [5,6,18] and are summarized in Table 3. Patients experiencing an acute anaphylactic reaction may say they feel odd or uncomfortable, or express a need to urinate or defecate. Such relatively nonspecific symptoms should be taken seriously and the patient evaluated by measuring blood pressure and pulse rate.
Once anaphylaxis is suspected, the patient’s airway, breathing, circulation, and level of consciousness should be assessed. If blood pressure is depressed, the patient should immediately be placed in the Trendelenburg position and an injection of epinephrine given intramuscularly into the lateral thigh muscle (vastus lateralis). A study of epinephrine plasma levels following intramuscular injection into the arm and into the leg compared to subcutaneous injection demonstrated the importance of injecting epinephrine into the lateral thigh muscle.[19] The typical dose of epinephrine is 0.3 mg and can be repeated every 5 to 15 minutes. While administration of intramuscular epinephrine will be sufficient treatment for many patients with anaphylaxis, failure of a prompt response should be quickly followed by establishing IV access for administration of normal saline and epinephrine.
For intravenous epinephrine administration, a concentration of 10 μg/mL (1:100,000), given by infusion and titrating the dose depending on the response and side effects, is the recommended treatment.[18] Figure 2 shows the need for frequent bolus dosing with intravenous epinephrine in patients with serious anaphylaxis. The use of continuous infusion provides the ability to titrate administration according to need (see legend to Figure 4). Normal saline should be given promptly, and rapid infusion of 1,000 mL over 1 to 3 minutes is recommended, repeating this dose as needed. Oxygen should be administered because of the known occurrence of hypoxia.
Studies in anesthetized ragweedsensitized dogs showed that epinephrine treatments by subcutaneous, intramuscular, and intravenous bolus were ineffective in preventing cardiovascular collapse. In contrast, a titrated intravenous infusion produced a sustained improvement.[20] It is important to keep in mind that overtreatment with epinephrine can lead to serious epinephrine toxicity, as manifested by tachycardia, hypertension, and the possibility of myocardial infarction and stroke.
The treatments discussed above and summarized in Table 3 are the critical elements of therapy. Other therapies, such as atropine administration, should be reserved for patients with bradycardia.[15] Therapies such as administration of antihistamines, including both blockers of the H1 receptor and H2 histamine receptors can be considered, although anaphylaxis is dependent on numerous mediators that are not affected by these medications. Administration of glucocorticoids is not a critical aspect of the treatment of anaphylaxis and should be considered only after all of the therapies described in Table 3 have been implemented.
Patients receiving beta-blockers have shown increased severity of anaphylaxis or resistance to treatment. Should a patient experiencing anaphylaxis also be taking beta-blockers, one should contemplate administration of glucagon, which is considered to activate adenylate cyclase independent of the beta receptor.[5] Patients taking glucagon may vomit, so precautions for protecting the airway should be taken. Biphasic Anaphylactic Reactions After treatment of the acute anaphylactic reaction, an observation period of at least 4 to 6 hours is recommended for mild to moderate reactions. For serious reactions, one should consider a more prolonged observation or hospitalization. The rationale for the prolonged observation is the occurrence of persisting manifestations of anaphylaxis once the effects of epinephrine have worn off. Another concern is a biphasic reaction. A biphasic anaphylactic reaction is any reaction occurring after complete resolution of immediate symptoms.
Various series have shown frequencies of biphasic reactions ranging from 3% to 20%.[6] Currently, no reliable clinical indicators identify patients at risk from a biphasic reaction, although a biphasic reaction may be more likely after an initial severe reaction and possibly more likely when epinephrine administration has been delayed.[21] The majority of the delayed reactions are mild and present with essentially the same features as the immediate reaction. The literature shows that delayed reactions have occurred within 24 hours. Patients at risk for a biphasic reaction or a delayed anaphylactic reaction should carry personal epinephrine injection devices and be instructed in their use.
Diagnostic Aids
Biochemical mediators of anaphylaxis- including histamine, tryptase, carboxypeptidase A, prostaglandin D2, leukotrienes, and PAF-are released during the degranulation of mast cells and basophils.[4] As noted in Figure 3, measurement of plasma histamine is useful to gauge the severity of anaphylaxis.
Similarly, measurement of serum tryptase should be performed in patients experiencing anaphylaxis.[22] Figure 3 shows how rapidly plasma histamine increases. In contrast, serum tryptase levels peak at 60 to 90 minutes after onset and can persist up to 6 hours. A recent study shows that PAF (platelet-activating factor) is released during anaphylaxis (see Figure 2 in the article by Drs. Chung and O’Neil that begins on page 14 of this supplement),[8] and PAF serum levels rise with increasing severity of anaphylaxis. The level of PAF is likely dependent not only on release from producing cells, but also on the rate of degradation. PAF acetyl hydrolase degrades PAF, and levels of PAF acetyl hydrolase were strikingly lower in patients with fatal peanut anaphylaxis, suggesting that patients who are most susceptible to anaphylaxis may have reduced levels of this enzyme.
All of the diagnostic aids for anaphylaxis require storing of serum or plasma for subsequent analyses, and these results are valuable only in retrospect. Therefore, the physician treating acute anaphylaxis has no immediate laboratory gauge of severity, save possibly measurement of the hematocrit. An elevated hematocrit would alert the treating physician that significant percentages of total blood volume had been lost through the dilated vasculature.
Anaphylaxis and Other Hypersensitivity Reactions Following Administration of Monoclonal Antibodies for Treatment of Malignant Disease
Recent information suggests that increasing use of monoclonal antibodies to target cancer is associated with a considerable number of severe infusion reactions[23] that are disruptive of patient care.[24] In the case of cetuximab (Erbitux), a high prevalence of hypersensitivity reactions has been reported.[25] These reactions are reportedly due to IgE antibodies to cetuximab and, specifically, to galactose-α-1,3-galactose present on the recombinant cetuximab.[26] Therefore, oncologists should become knowledgeable regarding the pathophysiology as well as the treatments and diagnostic aids for anaphylaxis summarized herein.
If anaphylaxis develops in a patient receiving medication, administration of the medication should be immediately stopped, and the intravenous line utilized for treatment following the recommendations summarized in Table 3. It is imperative that centers administering monoclonal antibodies have the medications and equipment needed for the treatment of severe anaphylaxis. Further, multicenter trials of new monoclonal antibodies should include appropriate studies to investigate anaphylaxis and to characterize the pathophysiology of these reactions. A report on reactions to cetuximab recently published in the New England Journal of Medicine by Chung and colleagues elegantly illustrates the information that it is possible to glean from such investigations.[26]
Recently a patient suffered fatal angioedema following panitumumab administration (personal communication, Dr. Volker Wagner, Amgen, Inc). The reaction occurred after the fourth panitumumab administration in a 71-year-old female with a history of locally recurrent and metastatic squamous cell carcinoma of the tongue. The patient had experienced an episode of angioedema approximately 6 days following the third panitumumab dose, and angioedema was initially attributed to an antibiotic reaction. However, approximately 2 days after the fourth exposure to panitumumab, facial swelling reoccurred. She was hospitalized and developed progressive respiratory distress; intubation was declined, and she died the following morning.
This is the first report of such a reaction to panitumumab. The nature of this apparent hypersensitivity reaction is obscure. It occurred too late to be attributed to anaphylaxis but could be an atypical late-phase reaction; an Arthus reaction or a reaction to products of necrotic tumor cells are other possibilities. Nonetheless, this event signals the need for caution about potential delayed hypersensitivity reactions in the administration of panitumumab and the need for investigation of such reactions to establish a mechanism.
Note added in proof: Kemp et al, in a statement for the World Allergy Organization, stress the importance of using epinephrine for the treatment of anaphylaxis and believe that presently epinephrine is underutilized and that most of the reasons proposed to withhold its use are fl awed (Kemp SF, Lockey F, Simons FE: Epinepherine: The drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 63:1061-1070, 2008).
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
This supplement and associated publication costs were funded by Amgen.
Acknowledgment: The authors wish to acknowledge William Fazzone, PhD, from MediTech-Media, Ltd., supported by Amgen, for editorial assistance including formatting of the manuscript for submission.
Address all correspondence to:
Gerald J. Gleich, MD
4A330 School of Medicine
30 North 1900 East
Salt Lake City, UT 84132-2409
e-mail: gerald.gleich@hsc.utah.edu
References:
References
1. Cohen SG, Zelaya-Quesada M: Portier, Richet, and the discovery of anaphylaxis: A centennial. J Allergy Clin Immunol 110:331- 336, 2002.
2. The Nobel lectures in immunology. The Nobel Prize for Physiology or Medicine, 1913, awarded to Charles Richet. “In recognition of his work on anaphylaxis.” Scand J Immunol 31:375-388, 1990.
3. Simons FE: 9. Anaphylaxis. J Allergy Clin Immunol 121:S402-S407; quiz S420, 2008.
4. Schwartz LB: Effector cells of anaphylaxis: Mast cells and basophils. Novartis Found Symp 257:65-74; discussion 74-79, 98-100, 276-285, 2004.
5. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology: The diagnosis and management of anaphylaxis: An updated practice parameter. J Allergy Clin Immunol 115 (suppl 2):S483-S523, 2005.
6. Sampson HA, Munoz-Furlong A, Campbell RL, et al: Second symposium on the definition and management of anaphylaxis: Summary report-Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 117:391-397, 2006.
7. Brown AF, McKinnon D, Chu K: Emergency department anaphylaxis: A review of 142 patients in a single year. J Allergy Clin Immunol 108:861-866, 2001.
8. Vadas P, Gold M, Perelman B, et al: Plateletactivating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med 358:28-35, 2008.
9. Sicherer SH, Leung DY: Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects. J Allergy Clin Immunol 116:153-163, 2005.
10. Sicherer SH, Munoz-Furlong A, Sampson HA: Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol 114:159-165, 2004.
11. Amin HS, Liss GM, Bernstein DI: Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol 117:169-175, 2006.
12. Gilstad CW: Anaphylactic transfusion reactions. Curr Opin Hematol 10:419-423, 2003.
13. Schmidt AP, Taswell HF, Gleich GJ: Anaphylactic transfusion reactions associated with anti-IgA antibody. N Engl J Med 280:188- 193, 1969.
14. Smith PL, Kagey-Sobotka A, Bleecker ER, et al: Physiologic manifestations of human anaphylaxis. J Clin Invest 66:1072-1080, 1980.
15. Brown SG, Blackman KE, Stenlake V, et al: Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J 21:149- 154, 2004.
16. Hunt KJ, Valentine MD, Sobotka AK, et al: A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med 299:157- 161, 1978.
17. Brown SG, Wiese MD, Blackman KE, et al: Ant venom immunotherapy: A double-blind, placebo-controlled, crossover trial. Lancet 361:1001-1006, 2003.
18. Brown SG: Cardiovascular aspects of anaphylaxis: Implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol 5:359-364, 2005.
19. Simons FE, Gu X, Simons KJ: Epinephrine absorption in adults: Intramuscular versus subcutaneous injection. J Allergy Clin Immunol 108:871-873, 2001.
20. Mink SN, Simons FE, Simons KJ, et al: Constant infusion of epinephrine, but not bolus treatment, improves haemodynamic recovery in anaphylactic shock in dogs. Clin Exp Allergy 34:1776-1783, 2004.
21. Sampson HA: Anaphylaxis: Persistent enigma. Emerg Med Australas 18:101-102, 2006.
22. Schwartz LB: Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am 26:451-463, 2006.
23. Schwartzberg LS, Stepanski EJ, Fortner BV, et al: Retrospective chart review of severe infusion reactions with rituximab, cetuximab, and bevacizumab in community oncology practices: Assessment of clinical consequences. Suppor Care Cancer 16:393-398, 2008.
24. Colwell HH, Mathias SD, Ngo NH, et al: The impact of infusion reactions on oncology patients and clinicians in the inpatient and outpatient practice settings: Oncology nurses’ perspectives. J Infus Nurs 30:153-160, 2007.
25. O’Neil BH, Allen R, Spigel DR, et al: High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Onco1 25:3644-3648, 2007.
26. Chung CH, Mirakhur B, Chan E, et al: Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 358:1109-1117, 2008.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.