Androgen Deprivation Therapy: A Survival Benefit or Detriment in Men With High-Risk Prostate Cancer?
Androgen deprivation therapy (ADT) has been used in the management of prostate cancer for more than four decades. Initially, hormone therapy was given largely for palliation of symptomatic metastases. Following several randomized trials of patients with intermediate- to high-risk prostate cancer that demonstrated improvements in biochemical control and survival with the addition of ADT to external beam radiotherapy, there was a dramatic increase in the use of hormone therapy in the definitive setting. More recently, the safety of ADT has been questioned, as some studies have suggested an association of hormone therapy with increased cardiovascular morbidity and mortality. This is particularly worrisome in light of practice patterns that show ADT use extrapolated to situations for which there has been no proven benefit. In the setting of dose escalation with modern radiotherapy, in conjunction with the latest concerns about cardiovascular morbidity with ADT, the magnitude of expected benefit along with potential risks of ADT use must be carefully considered for each patient.
Androgen deprivation therapy (ADT) has been used in the management of prostate cancer for more than four decades. Initially, hormone therapy was given largely for palliation of symptomatic metastases. Following several randomized trials of patients with intermediate- to high-risk prostate cancer that demonstrated improvements in biochemical control and survival with the addition of ADT to external beam radiotherapy, there was a dramatic increase in the use of hormone therapy in the definitive setting. More recently, the safety of ADT has been questioned, as some studies have suggested an association of hormone therapy with increased cardiovascular morbidity and mortality. This is particularly worrisome in light of practice patterns that show ADT use extrapolated to situations for which there has been no proven benefit. In the setting of dose escalation with modern radiotherapy, in conjunction with the latest concerns about cardiovascular morbidity with ADT, the magnitude of expected benefit along with potential risks of ADT use must be carefully considered for each patient.
There is mounting evidence that androgen deprivation therapy (ADT) for prostate cancer carries significant health risks. General awareness of the unpleasant but typically tolerable side effects of androgen suppression is not lacking. Recent studies, however, have reported clinically significant cardiovascular morbidity and increased mortality, calling into question the safety of androgen suppression. In the mid-1990s there was a precipitous rise in the use of ADT in the face of emerging data indicating improved disease control, as well as preliminary results of a randomized trial that demonstrated an overall survival (OS) benefit with ADT.[1] What followed was a dramatic expansion in the role of hormone therapy-from primarily palliation of symptomatic metastases, to a component of definitive treatment. It is estimated that more than 600,000 men in the United States receive ADT.[2] A recent report on practice patterns suggested that almost 70% of hormone therapy is prescribed to patients for whom there is no proven benefit.[3]
FIGURE 1
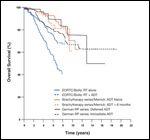
Overall survival of patients treated with radical prostatectomy, external beam radiotherapy and brachytherapy, stratified by androgen deprivation therapy use.
The role of androgen suppression in the primary treatment of prostate cancer is most clear for locally advanced disease. Patients with high-risk features (prostate-specific antigen [PSA] > 20 ng/mL, Gleason score ≥ 8, T3–4) are typically treated with external beam radiotherapy (EBRT) with ADT, radical prostatectomy, or prostate seed implant plus supplemental EBRT with or without ADT (Figure 1). It has now been more than 20 years since the standard use of ADT for locally advanced disease was first established. Given the latest concerns regarding hormone therapy, its overall effect on survival must be scrutinized. This review will re-examine the impact of ADT on survival as well as the available evidence in the context of issues that have come to light in the last two decades.
Modalities and Mechanism of Action
The Nobel Prize in medicine was awarded to Charles Huggins in 1966 for his discoveries regarding the androgen dependence of prostate cancer. Dr. Huggins’ work demonstrated that orchiectomy led to clinical improvement in patients with metastatic disease and, conversely, that androgen injections caused worsening of symptoms.[4] Androgens promote the growth of both benign glandular epithelial cells and malignant cells in the prostate. Depriving cancer cells of their stimulant causes regression that can be translated into symptomatic control in metastatic disease and possible improvement in cure rates in the definitive-treatment setting. After prolonged hormone therapy, prostate cancer can develop independence from androgens and become refractory to ADT.
Orchiectomy as the primary means of ADT has largely been replaced with pharmacologic agents because of both their reversibility and data from 10 randomized trials and meta-analysis showing equivalence to surgical castration.[5] Leuprolide and goserelin- gonadotropin-releasing hormone (GnRH) agonists, are the two most commonly used drugs. Under normal conditions, GnRH is released by the hypothalamus in a pulsatile fashion, stimulating the pituitary to secrete leutinizing hormone, which, in turn, promotes testosterone production in the testes. Over time, GnRH agonists induce downregulation of its receptors in the pituitary, thus leading to castrate levels of testosterone. Combined androgen blockade can be achieved by adding anti-androgens-bicalutamide or flutamide, which act directly at the prostate gland and decrease the adrenal contribution of androgens. In conjunction with radiotherapy, ADT is thought to reduce disease burden and therefore augment cell kill and improve tumor control. Androgen suppression has also been shown to promote apoptosis[6,7] and decrease hypoxia,[8] thereby producing a synergistic effect with concurrent ionizing radiation. In vivo experiments have also shown induction of an immune response.[9] Systemically, ADT may serve to eliminate micrometastases. The biologic mechanism of androgen deprivation in combination with radiotherapy and their interactions have not been fully elucidated. Further clarity is expected with ongoing investigations.
Although there are clear advantages to the reversibility of drugs and dampened psycho-physical insult compared with orchiectomy, the development of pharmacologic therapies with its financial incentives and ease of use has led to cavalier and inappropriate application, as evidenced by the millions of men who have received androgen suppression for nonproven indications.[10,11] In Medicare patients alone, more than $1 billion was spent on GnRH agonists in 2001, leading to a federal act instituted in 2004 that cut reimbursement such that, by 2005, reimbursement for GnRH agonists had decreased 40% to 50% compared with that in 2003.[12] Notably, from 2004 to 2005, coinciding with the year following the mandate to reduce reimbursement, there was a decline in the use of GnRH agonists and a corresponding increase in surgical castration, although not of equal magnitude.[12] One can hope that this imbalance is a result of more judicious ADT use and not a consequence of withholding proper treatment in the face of diminishing financial returns.
TABLE 1

Phase III Randomized Trials Investigating the Role of Androgen Deprivation Therapy With External Beam Radiotherapy in High-Risk Patients
Androgen Deprivation Therapy and External Beam Radiotherapy
Numerous phase III randomized trials have been completed investigating the role of ADT with EBRT in high-risk patients (Table 1). Three trials demonstrated an OS benefit and all studies found an improvement in prostate cancer mortality-these trials form the basis for the current standard use of ADT in the high-risk setting. One of the landmark studies was a European collaborative trial that randomized 415 patients with high-grade and/or T3–4 prostate cancer to EBRT alone or EBRT plus concurrent and adjuvant ADT for 3 years.[13] At 5 years, there was a 26% statistically significant OS benefit in patients who received hormone therapy. Recent key updates of two Radiation Therapy Oncology Group (RTOG) studies provided 10-year outcomes that remarkably also showed improvement in OS of 26%.[14,15] Given that it has been 10–20 years since the inception of these trials, it is important to note the changes in practice over this period in order to appropriately apply these data to current times. First, with the exception of one trial,[16] these studies primarily used clinical staging as eligibility criteria and represent a more locally advanced group than what is commonly diagnosed today as high-risk. For example, in the EORTC trial, 91% of patients had T3–T4 tumors and 33% had PSA > 40ng/mL[13]; currently this currently this is not a typical presentation. A more common high-risk patient in the modern era is one with a moderately elevated PSA, normal digital rectal exam, and Gleason score of 8; such a patient was in the minority of historic trials. In the setting of gross tumor and higher disease burden seen in earlier studies, ADT likely played a more critical role as EBRT alone at conventional doses was likely to be insufficient.
The second major change in the landscape of prostate cancer is that in recent years, a number of dose escalation trials have shown improvement in biochemical control rates with radiation doses greater than 70 Gy,[17-20] and as a result, the doses of 65–70 Gy utilized in all of the ADT trials are considered inadequate by today’s standards. Therefore, there is debate regarding the role of androgen suppression with higher doses of radiation, and the question arises as to whether ADT was simply compensating for inadequate dose. Retrospective data have suggested that the benefit of an escalated dose is greater than that from addition of ADT to conventional doses, suggesting that ADT cannot replace dose escalation.[21] To date, however, there has been no survival benefit shown with dose escalation, so one can argue that even with increasing radiation doses, ADT may still confer an additional benefit in highly selected patients. This question remains to be answered in a randomized trial.
Androgen Deprivation Therapy and Brachytherapy
The use of prostate seed brachytherapy has historically been reserved for patients with low- to intermediate-risk prostate cancer, as early outcomes of high-risk disease treated with brachytherapy were poor.[22] However, this was prior to the era of rigorous dosimetric cut-points and coverage of periprostatic tissue. Numerous modern series from high-volume institutions have now demonstrated superior control rates with the use of brachytherapy.[23-25] One must bear in mind the more favorable disease profile of high-risk brachytherapy series compared with EBRT studies; in the largest series, average Gleason scores were 7 to 8 and PSA was 12–15 ng/mL.[24,26] ADT is commonly used prior to brachytherapy for the purpose of reducing prostate size to improve the technical feasibility and side effect profile of brachytherapy, but it is not routinely given to achieve better tumor control. There has been no randomized trial investigating the impact of ADT on outcomes in conjunction with brachytherapy, and thus we currently rely on retrospective series to guide management.
Merrick et al. reported on 204 high-risk patients treated with pelvic EBRT followed by brachytherapy; they found that men treated with ADT had a significant improvement in biochemical control, with 10-year biochemical progression-free survival of 80% in hormone-naive patients, compared with 90%–95% in patients who received ADT.[24] This provides some evidence that even with ablative radiation dose to the prostate, there may still be benefit from ADT in patients with high-risk disease. There was no difference in cause-specific survival (CSS) or OS, although with long-term CSS rates greater than 90%, it would likely require a large sample size to detect a difference. A published series of intermediate- to high-risk patients treated at Mount Sinai Medical Center also showed improvement in biochemical control with the addition of neoadjuvant and adjuvant ADT; 5-year freedom from biochemical failure (FBF) for high-risk patients was 74% with ADT, vs a dismal 46% without it.[26] There was no difference among all patients who received a good-quality implant, however, with a 5-year FBF of 80% with or without ADT, whereas in patients who received a low implant dose, the 5-year FBF was 79% vs 38% (P = .0037). Patients were not further subdivided based on disease risk to assess the impact of both dose and androgen suppression specifically in high-risk patients; however, in high-risk patients who received both ADT and a high-dose implant, the reported 4-year FBF was 77%. In multivariate analysis, ADT use was the most significant predictor of biochemical control in both the high-risk patients and low-dose group. A recent multicenter analysis investigating the impact of radiation dose noted a significant improvement in 5-year biochemical control with ADT, from 77.5% to 96% (P = .001) in patients who received high-dose implants.[27] Longer follow-up is necessary to confirm these results and allow for restoration of testosterone levels.
There are conflicting data regarding use of ADT with brachytherapy, with some evidence of biochemical improvement in high-risk patients but no survival benefit; this further supports the theory that with adequate radiation dose, the benefit of ADT may be lost, although longer follow-up is needed. To help answer this question, there is an ongoing multi-institutional randomized trial investigating ADT with brachytherapy in high-risk patients; however, the trial may close early without results, as accrual is poor.
Androgen Deprivation Therapy and Radical Prostatectomy
Historically, radical prostatectomy (RP) has not been the treatment of choice for high-risk prostate cancer, primarily due to concerns regarding the ability to obtain clear margins in locally advanced disease. With the use of PSA and earlier detection, the contemporary cohort of high-risk patients consists less of those with bulky disease and more of patients with higher-grade tumors, making some patients appropriate candidates for surgery. At least 10 randomized trials have been performed investigating the role of ADT in conjunction with RP; however, most of these studies largely comprised of patients with early-stage disease. Some investigations showed improvement in pathologic findings, with reports of fewer positive margins, histologic down-grading, and decreased rates of lymph node involvement.[28-31] Despite improved pathologic outcomes, this did not consistently translate into any difference in disease control. No trials demonstrated an improvement in overall survival, with the exception of a study published by Messing et al.[32] that randomized 98 pathologically node-positive patients to immediate vs deferred ADT after RP. Ten-year OS was 75% vs 50% (P = .04) and CSS was 85% vs 53% (P = .0004), favoring immediate ADT. It is noteworthy that patients in the delayed arm were not treated until development of clinically evident metastases.
ADT has been incorporated into the treatment regimen in numerous trials assessing the utility of chemotherapy as part of multimodality therapy for high-risk disease. Southwest Oncology Group performed a phase III study (S9921) comparing adjuvant ADT after RP with ADT plus six cycles of mitoxantrone and prednisone. TAX-3501 was a three-arm randomized trial comparing surveillance, adjuvant ADT, and adjuvant ADT plus docetaxel in high-risk patients. Both studies closed early due to poor accrual. CALGB 90203, an ongoing trial, is investigating neoadjuvant ADT with docetaxel vs RP alone. In summary, outside of node-positive disease, there are no data supporting the use of ADT in conjunction with RP.
Timing and Duration
Both American[33] and European[34] groups conducted trials comparing long-term and short-term ADT with EBRT after earlier studies showed benefit with long-term ADT. In RTOG 92-02, men with clinical T2c–T4 disease were randomized to 4 months of neoadjuvant and concurrent ADT or 2 years total of ADT.[33] More than 50% of men had T3–4 disease and 33% had PSA > 30 ng/mL. There was a significant improvement in 10-year CSS and biochemical failure, but no difference in OS. An OS benefit was detected in a subset analysis of patients with Gleason scores of 8 to 10; the 10-year OS rate was 45% for long-term ADT vs 32% for short-term ADT (P = .006). EORTC 22961 compared 6 months vs 3 years of concurrent and adjuvant treatment, with the goal of showing noninferiority of shorter course ADT.[34] Seventy-five percent of patients had T3–4 disease and median PSA was 19 ng/mL. After median follow-up of 6 years, patients who remained on ADT for 3 years had statistically significantly improved OS, CSS, and biochemical control. Thus, the existing data suggest that very-high-risk, locally advanced patients seen more commonly in an earlier era, long-term ADT of 2–3 years with EBRT to 65–70 Gy leads to improved overall survival.
In the aforementioned retrospective analysis of patients treated with brachytherapy and supplemental EBRT,[24] there was no difference seen between less than 6 months vs more than 6 months of ADT. This may be a reflection of response in patients with a more favorable risk profile; that is, longer-term ADT does not confer additional benefit in this group. Based on this finding, a shorter course may be adequate with brachytherapy.
The optimal timing of androgen suppression is not entirely clear, as the key EBRT trials used adjuvant; neoadjuvant and concurrent; concurrent and adjuvant; as well as neoadjuvant, concurrent, and adjuvant regimens. The interaction of androgen suppression with radiation is complex, as evidenced by the results of RTOG 94-13,[35] which showed survival improvement with neoadjuvant and concurrent ADT with pelvic radiation, but a survival detriment with short-term adjuvant ADT. Given the observed in vitro synergistic effects, most would advocate for concurrent ADT with EBRT, if it is to be given.
Adverse Effects and Their Impact on Survival
For most men, ADT has an impact on quality of life, and for some, the adverse effects of ADT are significant enough to cause them to discontinue therapy before completing the recommended length of treatment. Numerous adverse effects result from induced hypogonadism-sexual dysfunction, hot flashes, weight gain, mood lability, sleep disturbance, gynecomastia, shrinkage of genitalia, decrease in bone density, depression, and cognitive decline.
More recently, there has been heightened concern regarding adverse physiologic alterations that can impact survival. Androgen suppression alters body composition by both decreasing lean body mass and increasing fat mass, even in the setting of short-term androgen suppression.[36-39] It has also become evident that there is a direct relationship between low testosterone level and decreased insulin sensitivity that is supported by improvement with testosterone replacement in hypogonadal men.[40] ADT initially induces hyperinsulinemia, which maintains euglycemia; however, hyperglycemia and frank diabetes develop with prolonged androgen suppression.[41] In one study, 44% of men who received ADT for 12 months or longer developed diabetes, compared with 12% of hormone-naive men.[42] There are conflicting data on the impact of ADT on lipid profile-some studies have shown elevated LDL and triglycerides[41] while others found no difference, but there is consistency in increased total cholesterol and HDL across studies.[43] Because of observed increases in HDL,[44,45] it is not entirely clear how the overall change in lipid profile impacts cardiovascular disease. Nonetheless, the constellation of these physiologic changes comprises the metabolic syndrome, which is associated with increased risk of cardiovascular disease and diabetes.[46] In a cross-sectional study, 55% of men who had received more than 12 months of ADT met the criteria for metabolic syndrome, compared with 20%–22% in the non-ADT and control groups.[41]
Because of these adverse physiologic changes, a number of recent studies have been performed to evaluate the incidence of cardiovascular disease in androgen-suppressed patients. The impact of these findings, specifically on mortality, will be reviewed here. A population-based study of more than 70,000 men with nonmetastatic prostate cancer found that rates of incident diabetes, incident coronary artery disease, myocardial infarction, and sudden cardiac death were all incrementally higher in men treated with GnRH agonists.[47] Another cohort of more than 19,000 men over 65 years of age who were treated with ADT for at least 6 months was matched with men with prostate cancer who had not received ADT. This cohort study showed that ADT was associated with increased diabetes and fragility fracture but not with acute myocardial infarction or sudden cardiac death.[48] D’Amico et al. performed a pooled analysis of three aforementioned trials, to evaluate the incidence of fatal myocardial infarction; they found that in men 65 years of age or older, 3–8 months of ADT was associated with an increased incidence of fatal myocardial infarctions, compared with men treated with radiotherapy alone.[49] There was not a sufficient number of events in patients younger than 65 years old for the investigators to draw any conclusions about this age group. Similar results were reported from an analysis of a prostate cancer registry; in patients 65 years or older treated with definitive radiation, there was only a trend toward a higher 5-year estimate of cardiovascular death with ADT: 8.4% vs 5.7% without ADT (P = .20), but there was a statistically increased risk of cardiovascular death in patients of all ages treated with radical prostatectomy and ADT.[50] Careful examination of the data reveals that many of the cardiovascular events occurred years after discontinuation of ADT-either a reflection of unbalanced baseline cardiovascular risk factors or the latency and irreversibility of the early effects of ADT. Testosterone recovery time can take 8–18 months, during which time patients are still at risk for toxicities.[51] It is noteworthy that in recent years, owing to advances in medical management, mortality from cardiovascular disease has declined and therefore the actual detriment in survival may be lower than previously reported.
Given the recent heightened awareness of cardiovascular impact, data on this particular effect have been reviewed for the landmark randomized trials. A multivariate analysis of patients enrolled on RTOG 85-13 showed that ADT was not associated with an increased risk of cardiovascular mortality (HR 0.99; 95% CI 0.58–1.69), with a 9-year cardiovascular mortality of 8% vs 11% with or without ADT (P = .17), respectively.[52] In an update of RTOG 86-10, there was no statistically significant difference in 10-year rates of cardiovascular deaths-12.5% with ADT vs 9% without ADT (P = .32).[15] An updated analysis of RTOG 92-02 was performed that found the 5-year rate of cardiovascular mortality was approximately 5% with both long- and short-term ADT and 10% in the subset of patients with a history of cardiovascular disease.[53] Data from EORTC 22961 showed no difference in cardiovascular disease between patients treated with 6 months vs 3 years of ADT.[34] These were all post hoc analyses and, as such, have their limitations. In future trials, it will be worthwhile to note the proportion of patients in each arm who were treated with ADT for disease recurrence, as this will also contribute to the incidence of morbidity through the follow-up period.
Increased risk of fracture secondary to ADT may also contribute to the survival equation when balancing the risks and benefits of treatment. Androgen suppression has been shown in numerous studies to decrease bone mineral density.[54-56] ADT for prostate cancer is now one of the leading causes of osteoporosis in this country.[2] Large population-based retrospective series have demonstrated an increased risk of fracture in men who received ADT.[57,58] Although the cause of increased fracture risk is also due to greater fall risk secondary to metastatic disease and treatment-related frailty, decreased bone density from prolonged androgen suppression is certainly a major contributor. It is well-accepted that hip fractures in the elderly have an impact on survival; similarly, aside from the obvious associated morbidity, skeletal fractures in men with prostate cancer have also been shown to increase mortality.[59]
There is some evidence that there may be a survival detriment with the use of ADT. A post-hoc subset analysis of the D’Amico trial suggested that in patients with moderate to severe comorbidity, the addition of ADT decreased OS, with 8-year estimates of 54% vs 25%, although the difference was not statistically significant (P = .08). In addition, this subset consisted of only 25 patients in each arm, hardly a sufficient number from which to draw firm conclusions. Furthermore, 71% of study participants had intermediate-risk disease, for which the benefit of ADT is likely to be smaller. An OS detriment with addition of ADT was found in a retrospective study of more than 2,000 consecutive patients representing all risk groups treated with brachytherapy, although patients in the study were notably older, with a median age of 73 years, compared with 65–68 years in other series. Subset analysis of high-risk patients was not performed.[60] Most recently, a retrospective study of more than 5,000 patients, comprising primarily low- to intermediate-risk patients treated with brachytherapy, showed that neoadjuvant ADT was associated with an increase in all-cause mortality in men with previous congestive heart failure or myocardial infarction.[61] Results of these studies and the D’Amico trial may be indication that in patients without clear benefit, for example, men at intermediate risk or treated with ablative doses, ADT is not beneficial and may be harmful. Future prospective trials that collect and stratify all patient factors with a significant impact on cardiovascular morbidity and mortality are necessary, to accurately define the subset of patients for whom the benefit of ADT outweighs its risks.
Summary
In his Nobel Prize address given a little over 40 years ago, Dr. Charles Huggins stated, “Mostly man with cancer lives 1 year or a little longer after the neoplasm becomes manifest, and it would appear that some inhibition of growth of the tumor takes place to produce this protracted course.” It is of great comfort and encouragement that our field has made immense strides in the last decades and “this protracted course” is now longer than a single year. However, the responsibility that comes with improving survival is mitigating long-term adverse effects of treatment and maintaining an acceptable quality of life.
Reference Guide
Therapeutic Agents
Mentioned in This Article
Bicalutamide
Docetaxel (Taxotere)
Flutamide
Goserelin
Leuprolide
Mitoxantrone
Prednisone
Testosterone
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Use of ADT in conjunction with EBRT at historical doses of 65–70 Gy in high-risk patients portends an OS benefit, as demonstrated in several large randomized controlled trials with some evidence that long-term ADT provides superior OS in very-high-risk, locally advanced disease. This has been further validated in a recent meta-analysis that included the aforementioned trials.[62] It remains to be seen whether ADT will provide additional benefit in the modern era of escalated radiation doses. In the meantime, it is imperative that we bear in mind the role of dose escalation when making management decisions based on historical trials. No survival advantage has been shown when androgen suppression is added to radical prostatectomy, with the exception of node-positive disease. There are no randomized studies evaluating its role with prostate brachytherapy-retrospective analyses have demonstrated significant improvement in biochemical control but no difference in survival.
It is of concern that practice patterns have been shown to be driven by inappropriate extrapolation of existing data and financial incentives.[3,12] This is particularly worrisome in light of growing evidence that ADT may be detrimental to the survival of our patients. Recent available data suggest possible increased cardiovascular morbidity and mortality associated with androgen suppression that must not be taken lightly. Given the large number of men receiving ADT, this is a serious health concern. ADT should be used discriminately and strictly in settings in which a clear benefit has been shown. Only in the rigorously selected patient will the benefit of ADT outweigh its risks. Modifiable cardiac risk factors ought to be addressed when initiating ADT, and special attention should be paid to patients’ global health status, to insure that we are not harming patients in the pursuit of improving cure rates and other less noble causes.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Bolla M, Gonzalez D, Warde P, et al: Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 337:295-300, 1997.
2. Smith MR: Androgen deprivation therapy for prostate cancer: New concepts and concerns. Curr Opin Endocrinol Diabetes Obes 14:247-254, 2007.
3. Shahinian VB, Kuo YF, Freeman JL, et al: Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol 25:5359-5365, 2007.
4. Huggins C, Hodges CV: Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 22:232-240, 1972.
5. Seidenfeld J, Samson DJ, Hasselblad V, et al: Single-therapy androgen suppression in men with advanced prostate cancer: A systematic review and meta-analysis. Ann Intern Med 132:566-577, 2000.
6. Kyprianou N, English HF, Isaacs JT: Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res 50:3748-3753, 1990.
7. Joon DL, Hasegawa M, Sikes CI, et al: Supraadditive apoptotic response of R3327-G rat prostate tumors to androgen ablation and radiation. Int J Radiat Oncol Biol Phys 38:1071-1077, 1997.
8. Nakashima J, Imai Y, Tachibana M, et al: Effects of endocrine therapy on the primary lesion in patients with prostate carcinoma as evaluated by endorectal magnetic resonance imaging. Cancer 80:237-241, 1997.
9. Mercader M, Bodner BK, Moser MT, et al: T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 98:14565-14570, 2001.
10. Cooperberg MR, Grossfeld GD, Lubeck DP, et al: National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst 95:981-989, 2003.
11. Shahinian VB, Kuo YF, Freeman JL, et al: Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 103:1615-1624, 2005.
12. Weight CJ, Klein EA, Jones JS: Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer 112:2195-2201, 2008.
13. Bolla M, Collette L, Blank L, et al: Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 360:103-106, 2002.
14. Pilepich MV, Winter K, Lawton CA, et al: Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 61:1285-1290, 2005.
15. Roach M, 3rd, Bae K, Speight J, et al: Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol 26:585-591, 2008.
16. D’Amico AV, Chen MH, Renshaw AA, et al: Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA 299:289-295, 2008.
17. Dearnaley DP, Sydes MR, Graham JD, et al: Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol 8:475-487, 2007.
18. Kuban DA, Tucker SL, Dong L, et al: Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 70:67-74, 2008.
19. Peeters ST, Heemsbergen WD, Koper PC, et al: Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24:1990-1996, 2006.
20. Zietman AL, DeSilvio ML, Slater JD, et al: Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 294:1233-1239, 2005.
21. Nguyen KH, Horwitz EM, Hanlon AL, et al: Does short-term androgen deprivation substitute for radiation dose in the treatment of high-risk prostate cancer? Int J Radiat Oncol Biol Phys 57:377-383, 2003.
22. D’Amico AV, Whittington R, Malkowicz SB, et al: Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969-974, 1998.
23. Dattoli M, Wallner K, True L, et al: Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and high-risk features. Cancer 110:551-555, 2007.
24. Merrick GS, Butler WM, Wallner KE, et al: Androgen deprivation therapy does not impact cause-specific or overall survival in high-risk prostate cancer managed with brachytherapy and supplemental external beam. Int J Radiat Oncol Biol Phys 68:34-40, 2007.
25. Stock RG, Ho A, Cesaretti JA, et al: Changing the patterns of failure for high-risk prostate cancer patients by optimizing local control. Int J Radiat Oncol Biol Phys 66:389-394, 2006.
26. Lee LN, Stock RG, Stone NN: Role of hormonal therapy in the management of intermediate- to high-risk prostate cancer treated with permanent radioactive seed implantation. Int J Radiat Oncol Biol Phys 52:444-452, 2002.
27. Stone NN, Potters L, Davis BJ, et al: Multicenter analysis of effect of high biologic effective dose on biochemical failure and survival outcomes in patients with Gleason Score 7-10 prostate cancer treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 73:341-346, 2009.
28. Aus G, Abrahamsson PA, Ahlgren G, et al: Three-month neoadjuvant hormonal therapy before radical prostatectomy: A 7-year follow-up of a randomized controlled trial. BJU Int 90:561-566, 2002.
29. Meyer F, Moore L, Bairati I, et al: Neoadjuvant hormonal therapy before radical prostatectomy and risk of prostate specific antigen failure. J Urol 162:2024-2028, 1999.
30. Soloway MS, Pareek K, Sharifi R, et al: Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol 167:112-116, 2002.
31. Schulman CC, Debruyne FM, Forster G, et al: 4-year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol 38:706-713, 2000.
32. Messing EM, Manola J, Yao J, et al Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 7:472-479, 2006.
33. Horwitz EM, Bae K, Hanks GE, et al: Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26:2497-2504, 2008.
34. Bolla M, de Reijke TM, Van Tienhoven G, et al: Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360:2516-2527, 2009.
35. Lawton CA, DeSilvio M, Roach M, 3rd, et al: An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys 69:646-655, 2007.
36. Basaria S, Lieb J, 2nd, Tang AM, et al: Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 56:779-786, 2002.
37. Lee H, McGovern K, Finkelstein JS, et al: Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer 104:1633-1637, 2005.
38. Berruti A, Dogliotti L, Terrone C, et al: Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol 167:2361-2367; discussion 2367, 2002.
39. Smith MR, Finkelstein JS, McGovern FJ, et al: Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 87:599-603, 2002.
40. Marin P, Holmang S, Jonsson L, et al: The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 16:991-997, 1992.
41. Braga-Basaria M, Dobs AS, Muller DC, et al: Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 24:3979-3983, 2006.
42. Basaria S, Muller DC, Carducci MA, et al: Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 106:581-588, 2006.
43. Nishiyama T, Ishizaki F, Anraku T, et al: The influence of androgen deprivation therapy on metabolism in patients with prostate cancer. J Clin Endocrinol Metab 90:657-660, 2005.
44. Dockery F, Bulpitt CJ, Agarwal S, et al: Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 104:195-201, 2003.
45. Smith MR, Lee H, Nathan DM: Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 91:1305-1308, 2006.
46. Isomaa B, Almgren P, Tuomi T, et al: Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24:683-689, 2001.
47. Keating NL, O’Malley AJ, Smith MR: Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24:4448-4456, 2006.
48. Alibhai SM, Duong-Hua M, Sutradhar R, et al: Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 27:3452-3458, 2009.
49. D’Amico AV, Denham JW, Crook J, et al: Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 25:2420-2425, 2007.
50. Tsai HK, D’Amico AV, Sadetsky N, et al: Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst 99:1516-1524, 2007.
51. Pickles T, Agranovich A, Berthelet E, et al: Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer 94:362-367, 2002.
52. Efstathiou JA, Bae K, Shipley WU, et al: Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol 27:92-99, 2009.
53. Efstathiou JA, Bae K, Shipley WU, et al: Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: Analysis of RTOG 92-02. Eur Urol 54:816-823, 2008.
54. Daniell HW, Dunn SR, Ferguson DW, et al: Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol 163:181-186, 2000.
55. Diamond T, Campbell J, Bryant C, et al: The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: Longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer 83:1561-1566, 1998.
56. Maillefert JF, Sibilia J, Michel F, et al: Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol 161:1219-1222, 1999.
57. Shahinian VB, Kuo YF, Freeman JL, et al: Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352:154-164, 2005.
58. Smith MR, Boyce SP, Moyneur E, et al: Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol 175:136-139; discussion 139, 2006.
59. Oefelein MG, Ricchiuti V, Conrad W, et al: Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 168:1005-1007, 2002.
60. Beyer DC, McKeough T, Thomas T: Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 61:1299-1305, 2005.
61. Nanda A, Chen MH, Braccioforte MH, et al: Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA 302:866-873, 2009.
62. Bria E, Cuppone F, Giannarelli D, et al: Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer? Meta-analysis of randomized trials. Cancer 115:3446-3456, 2009.
63. Denham JW, Steigler A, Lamb DS, et al: Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol 6:841-850, 2005.
64. Zwergel U, Suttman H, Schroeder T: Outcome of prostate cancer patients with initial PSA > or = 20 ng/ml undergoing radical prostatectomy. Eur Urol 52:1058-1065, 2007.