Antibody Test to be Used in Large Scottish Lung Cancer Screening Trial
Scotland will soon begin a large prospective trial of early screening for lung cancer in high-risk patients using a simple blood test. The test, called EarlyCDT-Lung, has been in use and trials in the United States for more than two years.
Scotland will soon begin a large prospective trial of early screening for lung cancer in high-risk patients using a simple blood test. The test, called EarlyCDT-Lung, has been in use and trials in the United States for more than 2 years.
According to the company that manufactures the test,
Early
CDT-Lung can detect about half the cancers in a screened population and will reduce the number of people who need to undergo CT scans to 7%.
EarlyCDT-Lung, made by Kansas City–based Oncimmune, tests blood autoantibodies against a panel of 6 tumor-related antigens: p53, NY-ESO-1, CAGE, GBU4-5, Annexin 1, and SOX2. Elevation of the antibodies to these proteins can suggest the presence of a tumor, up to 5 years before imaging procedures might find it.
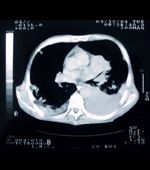
The new trial will include 10,000 participants who have smoked the equivalent of 20 cigarettes per day for more than 20 years; half will undergo testing with EarlyCDT-Lung followed by computed tomography scans if the blood test is positive, while the other half receive current standard of care treatment. The trial will start by the end of 2012, and results are expected by the end of 2014.
Screening for lung cancer has not achieved cost-effectiveness in the past, though there has been a move toward screening recently. The National Lung Screening Trial enrolled more than 53,000 high-risk individuals at 33 centers in the United States, and found that low-dose CT screening can reduce mortality from lung cancer. The rate of death from any cause was 6.7% lower in those who underwent CT screening compared with a standard radiography group. The rate of death due to lung cancer alone was reduced by 20%. There is no suggestion, though, that such screening can be cost-effective. The most recent recommendation from the U.S. Preventive Services Task Force, from 2004, says that the “evidence is insufficient” to recommend for or against screening with low-dose CT or other methods.
According to Oncimmune, EarlyCDT-Lung can detect about half the cancers in a screened population and will reduce the number of people who need to undergo CT scans to 7%. With half the cancers restricted to a 7% chunk of the overall screened group, the company thinks it will be a cost-effective screening method.
"This is a really important study,” said Frank Detterbeck, chief of thoracic surgery at Yale University School of Medicine in New Haven, Connecticut, in a press release from Oncimmune. “We know now that screening with CT imaging can save a lot of lives by early detection of lung cancer, but broad implementation of CT screening is not so easy. The significance of this blood test is the extensive and careful study, validation and revalidation that has produced consistent robust results, and the ease of use.”
Sir Harry Burns, the Chief Medical Officer for Scotland, said that the screening trial is part of the country’s Detect Cancer Early program; the aim of that program is to increase early detection of all cancers by 25%. “The earlier a cancer is diagnosed the greater the chance it can be treated successfully, and currently 85 percent of patients with lung cancer remain undiagnosed until the disease has reached an advanced stage,” he said in a press release.
A study of screening with EarlyCDT-Lung is also planned at the Mayo Clinic in Rochester, Minnesota; researchers hope to enroll 1,600 patients and complete the study by 2015.