ASCO 2011: Crizotinib Continues to Show Responses in Lung Cancer Patients
Results from an ongoing Phase II trial of patients with non-small cell lung cancer (NSCLC) that harbor a specific mutation in the anaplastic lymphoma kinase (ALK) gene reported at ASCO.
An ongoing Phase II trial of patients with non-small cell lung cancer (NSCLC) that harbor a specific mutation in the anaplastic lymphoma kinase (ALK) gene reported at ASCO that ~80% of trial participants have responded to crizotinib. A second presentation at ASCO showed that 61% of NSCLC patients showed a response in an expansion of the Phase I crizotinib study.
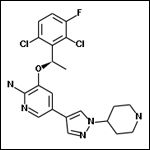
Structural diagram of crizotinib
Crizotinib is an oral, selective, small-molecule inhibitor developed by Pfizer. Recall that we reported on the publication of a portion of this Phase I trial in the New England Journal of Medicine (NEJM) ("Targeted Lung Cancer Drug, Crizotinib Shows Promise in Typically Unresponsive Non-Small-Cell Lung Cancer" Feb. 24, 2011).
Early Phase II Data
The preliminary data from the PROFILE 1005 trial consisted of 136 patients from 12 countries who had progressed on prior treatments and included patients with brain metastases. Most of these patients were heavily pre-treated with at least 2 prior chemotherapy regimens. 88% of the patients are still on the treatment after a median of 9 weeks of treatment. 83% of patients that were evaluable (76 of out 136) had lesion shrinkage, with 41 patients having more than 30% shrinkage. Seven patients have experienced progressive disease. The most frequent treatment adverse events are nausea, vision disorder, vomiting, and diarrhea that were mostly grade 1 and 2. Approximately 15% of patients have reported treatment-related toxicity. According to the study authors, crizotinib was " safe and well-tolerated with preliminary evidence of improved symptoms and clinically meaningful antitumor activity in pts with pre-treated ALK-rearranged."
Phase I Progression Free Survival Data
A second presentation at ASCO was the reporting of a continuation of the Phase I trial that was published in the NEJM by Dr. Ross Camidge from the University of Colorado. The median duration of response for this extended cohort was 8 weeks, with some patients exhibiting response as early as 2 weeks after the start of treatment. Median duration of follow-up was 11 months.
The overall response rate (complete and partial response) was 61% with 2 complete and 69 partial responses. Disease control rate, including complete and partial responses as well as stable disease, was 79% at 8 weeks and 67% at 16 weeks.The best response was from treatment-naive patients who had an 80% overall response compared with 60% for those who had received previous systemic therapies. The preliminary median progression free survival was 10 months. The median overall survival has not yet been reached, with no treatment-related study deaths reported. The best response was from treatment-naive patients who had an 80% overall response, compared with 60% for those who had received previous systemic therapies. The preliminary median progression free survival was 10 months.
Toxicity was reported as easily manageable with mostly grade 1 and 2 treatment-related toxicities consistent with those reported in the Phase II cohort. Toxicities generally diminished as treatment continued. The study authors describe the efficacy of crizotinib as "robust, rapid, durable and clinically meaningful"
Crizotinib Filed with the FDA
Pfizer had begun a rolling submission of a new drug application (NDA) to the FDA for NSCLC patients who harbor the ALK mutation in January of this year. The company announced that it has completed the filing with the FDA as well as the Japanese Ministry of Health, Labour, and Welfare in May. In the US, crizotinib has a priority 6-month review. Pfizer is highlighting the substantial response rate of crizotinib in patients that generally have a poor prognosis. According to the head of Pfizer's oncology business unit, the drug ""may change the treatment paradigm for patients with ALK-positive advanced NSCLC". Approximately 45,000 newly diagnosed NSCLC patients have the ALK fusion, or about 3-5% of all newly diagnosed NSLCL. Typically, these patients are non-smokers. A fraction of these patients will progress to advanced disease and will be eligible for crizotinib.
Targeted Therapeutic Test
Pfizer is currently collaborating with Abbott Molecular on a standardized test that will utilize FISH to detect patient tumors that are ALK-positive. The company is also exploring other options including a PCR test and immunohistochemistry in order to identify all eligible patients and allow easier testing in community settings.
Atypically Fast Progression from Clinical Trials to NDA Filing
Oncology drugs normally take at least 10 years to make it to commercial filing status, however, crizotinib has managed to do this in 5 years: Initial clinical trials began in 2006. Pfizer has stated that the filed NDA is based on early-stage trials and that they will not need to wait for completion of the currently ongoing Phase 3 crizotinib trials.
Current ongoing trials include a Phase 1b trial in tumor patients positive for the ALK mutation excluding NSCLC patients; a Phase II NSCLC single-arm 400 patient trial; and a randomized Phase 3 trial that compares crizotinib to the standard of care, docetaxel and pemetrexed in patients with advanced NSCLC. Additionally, the Memorial Sloan Kettering Cancer Center is running a trial to determine the mechanism of resistance in NSCLC patients with the ALK fusion gene by examining tumors from 40 patients to determine the genetic changes that occur that faciliate resistance to crizotinib.
All of this is good news for NSCLC patients as well as for the oncology field as a whole as it moves towards real personalized therapies based on the biology of a patient's tumor.