Bevacizumab/Erlotinib Regimen Produces Notable Clinical Benefits in Metastatic Renal Carcinoma
This supplement to Oncology News International includes 17 reportson clinical trials of targeted therapies used alone, in combination with chemotherapy,or in combination with each other in the treatment of non–small-cell lung cancer (NSCLC),bronchoalveolar carcinoma, glioblastoma multiforme, and renal cell carcinoma.Included is a report on a novel targeted agent recently approved for treatment of NSCLC.
NASHVILLE, Tennessee-Preliminary results of a phase II studyof bevacizumab (Avastin) and erlotinib(Tarceva) in patients with meta-static renal carcinoma show a substantialnumber of durable partial responsesand support further study of thisregimen, said John D. Hainsworth,MD, of the Sarah Cannon Cancer Center/Tennessee Oncology in Nashville(abstract 4502). "This tumor is refractoryto standard chemotherapy, andonly a small percentage of patientsbenefit from cytotoxic therapy," hetold Oncology News International.Dr. Hainsworth explained thatmost patients with clear cell renal carcinomahave loss of the VHL protein,resulting in overexpression of a numberof genes including vascular endothelialgrowth factor (VEGF), tumorgrowth factor (TGF)-beta, epithelialgrowth factor (EGF), and platelet derivedgrowth factor (PDGF). In second-line use, bevacizumab, an anti-VEGF antibody, prolongs time toprogression in advanced renal carcinoma."Based on renal carcinoma biology,we hypothesized that combinedVEGF and EGFR inhibition wouldenhance biological and clinical effects,"Dr. Hainsworth said.Original Protocol AmendedIn this phase II trial, bevacizumabwas combined with the EGFR antagonisterlotinib. The study enrolled patients with metastatic clear cell renalcarcinoma, and 0 or 1 previous systemicregimen. Patients were treatedwith bevacizumab at 10 mg/kg IVq 2 weeks and erlotinib at 150 mg/d po.
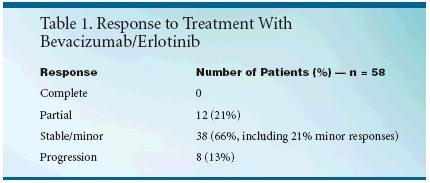
Patients were evaluated for responseafter 8 weeks. The investigators hadoriginally intended to continue treatmentfor 12 months, but the originalstudy protocol was amended to permitcontinuation in patients untildisease progression.Dr. Hainsworth reported data for58 treated patients with median follow-up of 11 months. In contrast toseveral other trials, 68% had no priortreatment. Eighteen percent had pre-paviousinterferon, 8% had previous interleukin-2 (IL-2) plus interferon, and6% had previous IL-2. Fifty three patientshad previous nephrectomies.Sites of metastases included lungs (41),liver (17), bone (15), adrenals (10),and other sites (14)."Nine of 12 patients with partialresponses and 10 of 12 with minorresponses [20% to 30% decrease intumor size] remain progression-free,"said Dr. Hainsworth (see Table 1). "Atotal of 42% of patients had measurabledecreases in their tumor size.Twenty six of the 38 stable or minorresponse patients remain alive and ontreatment for more than 6 months.""Responses have been seen in allareas of metastases and in most casesincluded reductions of more than 50%.For example, one patient had innumerablebilateral lung metastases thatcleared substantially with treatment, "Dr. Hainsworth said.Twelve MonthPFS of 50%Six-month progression-free survival(PFS) was 67%, and 12-month PFSwas 50%. Six-month overall survivalwas 92%, and 12-month overall survivalwas 81%.Treatment was tolerable in mostpatients but with a number of minorside effects, Dr. Hainsworth noted.Twenty-five patients (63%) had nograde 3 or 4 toxicity. Only two patientsstopped treatment because oftoxicity, both as a result of rash.Grade 3 or 4 toxicity included rash ineight patients (13%), diarrhea in six(10%), and nausea and vomiting in six(10%). Grade 1 or 2 bleeding occurredin 24 patients (39%) and grade 3 or 4bleeding was reported in 3 patients(5%)."The number of bleeding occurrencesmay seem out of proportion.We were somewhat concerned aboutbleeding, which has been a problemwith bevacizumab, but most instanceswere minor hemorrhoidal bleeding orminor nosebleeds. We feel that this isprobably not a true level of treatmentrelatedtoxicity," Dr. Hainsworth said."Most of the side effects were the rashand diarrhea typical of EGF receptorinhibitors."Good MajorResponse RateThe investigators concluded thatbevacizumab/erlotinib produced amajor response rate of 21% in thisgroup of 58 evaluable patients withadvanced renal carcinoma. An additional45% of patients had stable diseaseor minor response for more than6 months, and Dr. Hainsworth saidthat an increasing number of patientshave now maintained this result formore than 12 months with continuedtreatment. One-year PFS was 50%,and overall survival was 81%."This regimen appears to be one ofthe most active and best tolerated regimensin the treatment of advancedrenal carcinoma. The activity of thiscombination of two targeted agentsappears greater than the activity ofeither agent used alone in clear cellrenal carcinoma. Comparison of thisregimen to standard regimens is certainlyindicated. The results of thistrial also support further developmentof combinations of targeted agents toexploit tumor-specific biologicmechanisms in renal cancer and othermalignancies," Dr. Hainsworthconcluded.