Biomarkers to Identify the Risk of Metastasis in Melanoma Patients
A blood test could soon be used to predict the risk of the spread of cancer among melanoma patients. The study identifying the potential test was published online on April 12th in Clinical Cancer Research, and AACR journal.
A blood test could soon be used to predict the risk of the spread of cancer among melanoma patients. The study identifying the potential test was published online on April 12th in Clinical Cancer Research, an AACR journal. (DOI:10.1158/1078-0432.CCR-10-2402).
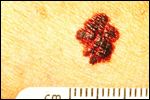
Seen is melanoma with a border that is uneven, ragged, or notched. Part of the ABCDs for detection of melanoma.
With diagnoses of melanoma rising and early-stage patients being actively monitored for disease recurrence, researchers at Yale University set out to identify a novel and more efficient way to monitor levels of melanoma-specific proteins in the blood of patients. The scientists identified a set of plasma biomarkers that could predict the risk of melanoma metastasis.
The Study Design
Blood samples were obtained from 108 patients with metastatic melanoma (MM) and 108 patients with stage I or stage II resected melanoma, matched by both age and gender. Healthy patients were also sampled to determine “normal” ranges of the biomarkers tested. The researchers selected potential blood markers that have been identified in previous scientific studies and implicated in skin cancer. Levels of each marker were compared between the three cohorts.
Study Results
The levels of seven markers were higher in patients with MM compared with those who had early-stage disease. The seven plasma biomarkers were CEACAM, ICAM-1, osteopontin, MIA, GDF-15, TIMP-1 and S100B. 81% of the stage I and II patients had no marker elevation and 69% of the MM had elevation of at least one marker (P < .0001). None of the markers were associated specifically with any age group or gender. Considering elevated levels of at least two markers as “abnormal,” the sensitivity of the assay among all patients was 57.4% with a specificity of 94.4%.
A measure of the test’s reliability, on a scale from 0.5 to 1.0 with 1 being optimal and 0.5 being useless, showed the assay had a reliability of 0.898. The increased reliability is attributed to using several biomarkers in combination.
Potential for a Widely Used Clinical Assay to Identify High Risk Relapse Patients
Patients with stage I to stage III melanoma have anywhere between a 10% and an 80% chance of death from melanoma. The actual risk depends on currently known prognostic variables.
Within each staging group, it is still very difficult to predict which patient is more susceptible to relapse, making patient monitoring, including physical exams, blood tests, and imaging crucial. While the NCCN recommends imaging for stage I patients only if symptoms are present and CT and PET scans for stage II, no universally followed monitoring algorithm exists. Likewise, there is no consensus on check-ups and blood tests among the melanoma community. However, tools for early detection of metastases and earlier treatment are crucial for improved overall survival, as has been shown in active surveillance studies in melanoma patients.
The impetus for the current study was to find a low-cost blood test that would substitute more costly imaging protocols in patients with early stage, resected disease. “The rate at which melanoma is increasing is dramatic, and there is a huge number of patients under surveillance,” stated one of the study authors, Harriet Kluger, MD. “Our current method of surveillance includes periodic imaging, which creates huge societal costs,” she added.
The targeted, goal-oriented study described here employed a genome-wide technique and will hopefully result in a cost-effective and sensitive clinical assay that allows efficient melanoma patient monitoring to identify those at high risk for relapse of disease.
"This finding will need to be confirmed prospectively before it is used in the clinic, but it shows that such testing is possible," said Dr. Kruger. After a prospective validation study, this plasma marker assay may complement the currently widespread use of lactate dehydrogenase (LDH) and imaging tests.