Bispecific Antibody Dose Schedules in Relapsed/Refractory Multiple Myeloma
A panel of experts spoke about the armamentarium of bispecific antibody therapies for patients with relapsed/refractory multiple myeloma and discussed step-up dosing considerations for these approved agents.
Ajai Chari, MD
University of California, San Francisco Helen Diller Family Comprehensive Cancer Center

Brea C. Lipe, MD
University of Rochester Medical Center

Binod Dhakal, MD, MS
Medical College of Wisconsin

Jeffrey V. Matous, MD
Colorado Blood Cancer Institute

At an Around the Practice® program hosted by CancerNetwork®, an expert panel discussed currently available treatment options for patients with relapsed/refractory multiple myeloma, particularly bispecific antibody therapies that have received approval from the FDA. They reviewed their strategies for administering these agents across various step-up doses and modifying treatment schedules following toxicity in the context of 2 patient cases. Ajai Chari, MD, a professor of clinical medicine and the director of the Multiple Myeloma Program at the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center, led the panel.
Other panelists included Brea C. Lipe, MD, a professor of medicine and the clinical director of the Multiple Myeloma program at the University of Rochester Medical Center; Binod Dhakal, MD, MS, an associate professor of medicine at Medical College of Wisconsin; and Jeffrey V. Matous, MD, a member physician at the Colorado Blood Cancer Institute and part of the Plasma Cell Diseases Group.
Patient Case 1 Managed by Binod Dhakal, MD

Chari: What are your thoughts on these patient cases? Would you have treated them any differently?
Lipe: No, these are great cases. With the first case, we’ve all had those patients whose diseases are hard to control. That’s where the excitement with some of these therapies comes from; it is not just that patients are doing well for a longer period. These patients for whom we historically didn’t have an armamentarium for can now receive therapies that we couldn’t give before. That was well exemplified by the first case.
Matous: We never had anything like this. We never had the opportunity to treat our patients with an effective therapy like this when they had these diseases. The first patient was one we would have allografted 10 to 15 years ago because we knew that patient was not going to have anything else available to them.
Dhakal: Now, I have a follow-up with my patient. He will be starting treatment with talquetamab-tgvs [Talvey] because he’s already progressing. We didn’t have any options before, but now talquetamab is approved for relapsed/refractory multiple myeloma.1 That would be a perfect choice for him. Otherwise, we would have given him a renal transplant. When he was having a good response, he had already seen a transplant doctor. Unfortunately, his disease progressed, so a transplant was placed on the back burner.
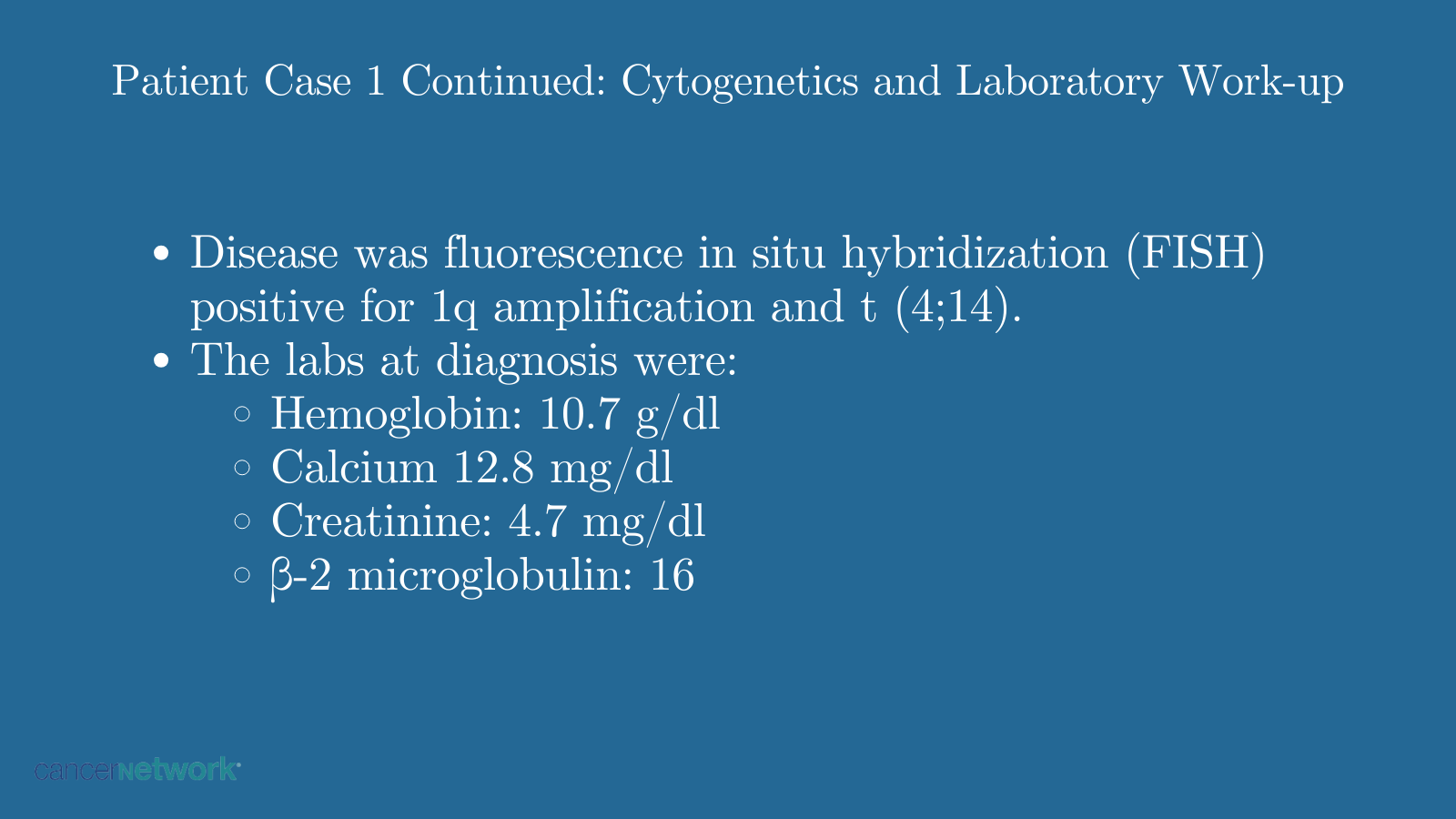
Patient Case 1 Continued: Cytogenetics and Laboratory Work-up
Bispecific Antibodies in Multiple Myeloma
Chari: What do the data show for currently approved B-cell maturation antigen [BCMA] bispecific therapies including teclistamab-cqyv (Tecvayli) and elranatamab-bcmm (Elrexfio)?2,3 What about the data for talquetamab? How have these agents changed your practice?
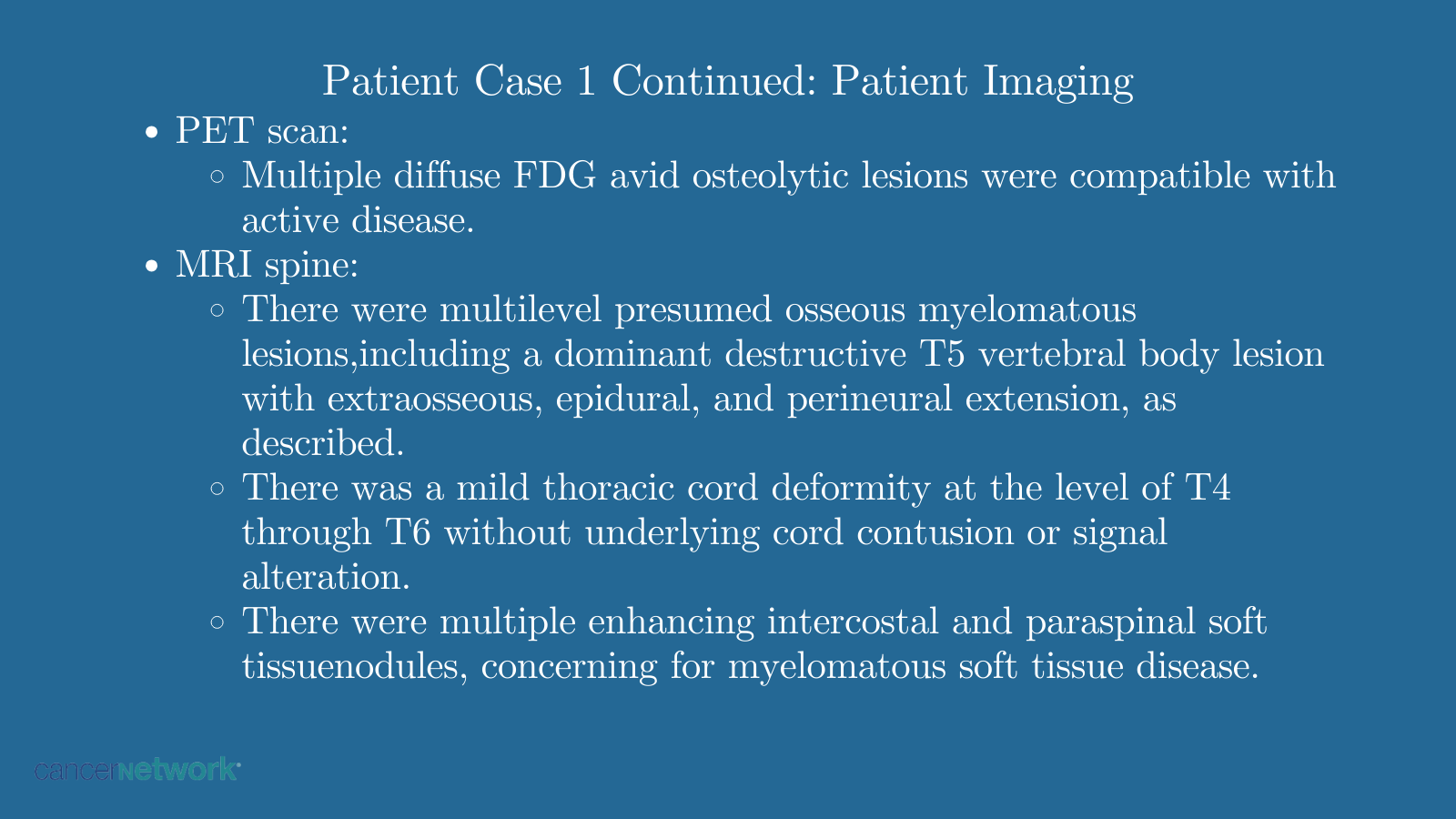
Patient Case 1 Continued: Patient Imaging
Dhakal: I don’t think managing my [patient] would have been possible if these drugs were not available. The cases demonstrate an unmet area where we don’t have very effective therapies. Having both BCMA- and non–BCMA-directed antibodies with very high response rates and a progression-free survival [PFS] in the ballpark of at least 12 months is exciting.
Chari: When a new drug gets approved, there’s a pretty big lag before you can get access to it. How quickly did you try to access these agents for your patients?
Lipe: We were in conversation way before the actual approvals for these agents about when our distributors were going to get them. As soon as we could get our hands on them, we had a line of patients.
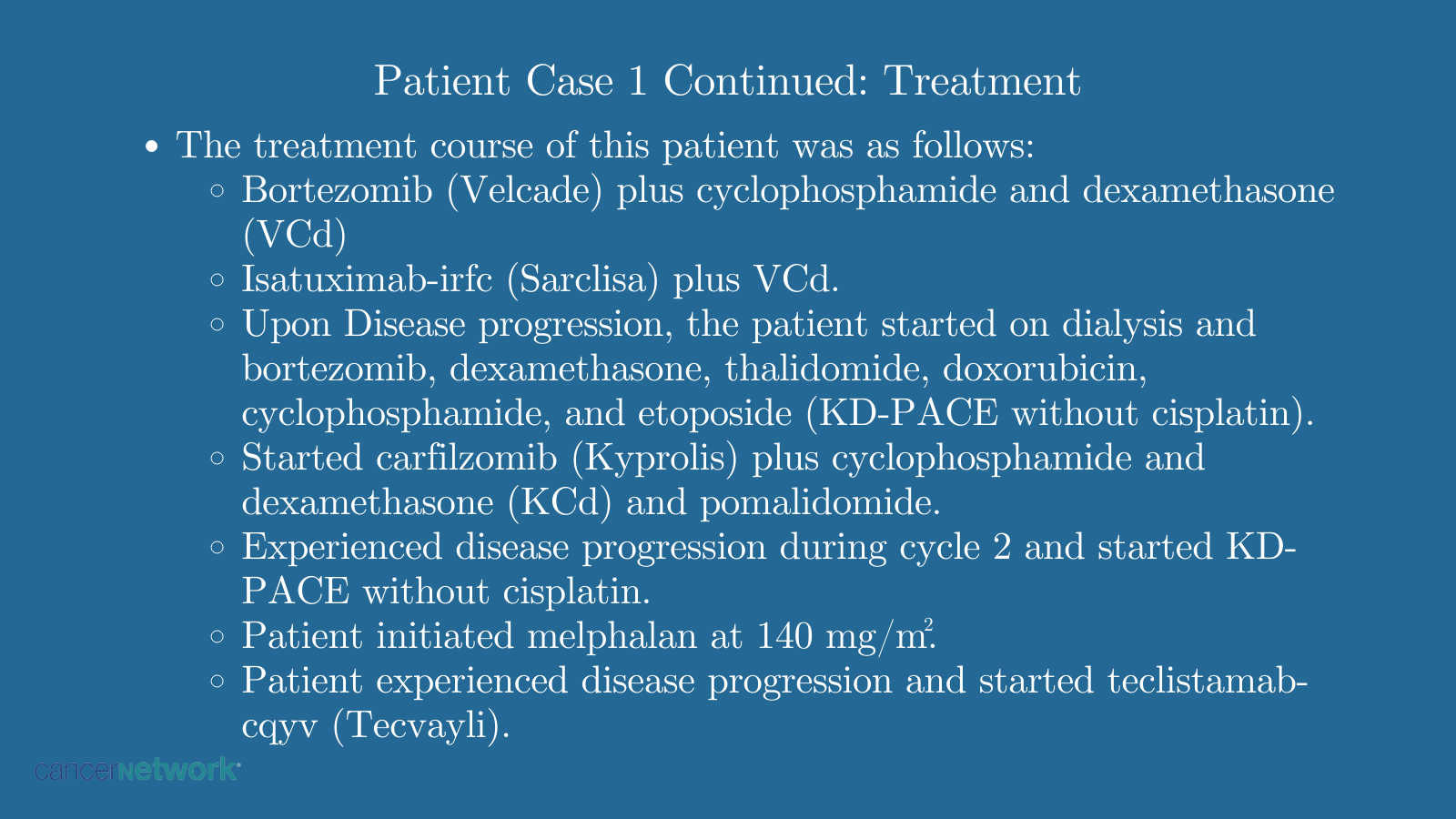
Patient Case 1 Continued: Treatment
Matous: I’m sure we all had patients that we had evaluated for clinical trials, and they weren’t eligible to get them at the moment. We couldn’t wait for commercial approval to be able to give these agents to more patients.
Lipe: One of the reasons it’s hard to get patients enrolled on clinical trials is when they have explosive disease. There’s often a couple of weeks between when you can put them on a trial and when they can get treatment. Patients who are progressing, which is not uncommon in this very heavily pretreated population, represented another need when clinical trials just aren’t going to cut it as their disease is exploding so quickly.
Chari: Are there any patients you would not offer a bispecific antibody to?
Dhakal: Right now, I can offer them to all the patients I see in the clinic.
Patient Case 1 Continued: Follow-up
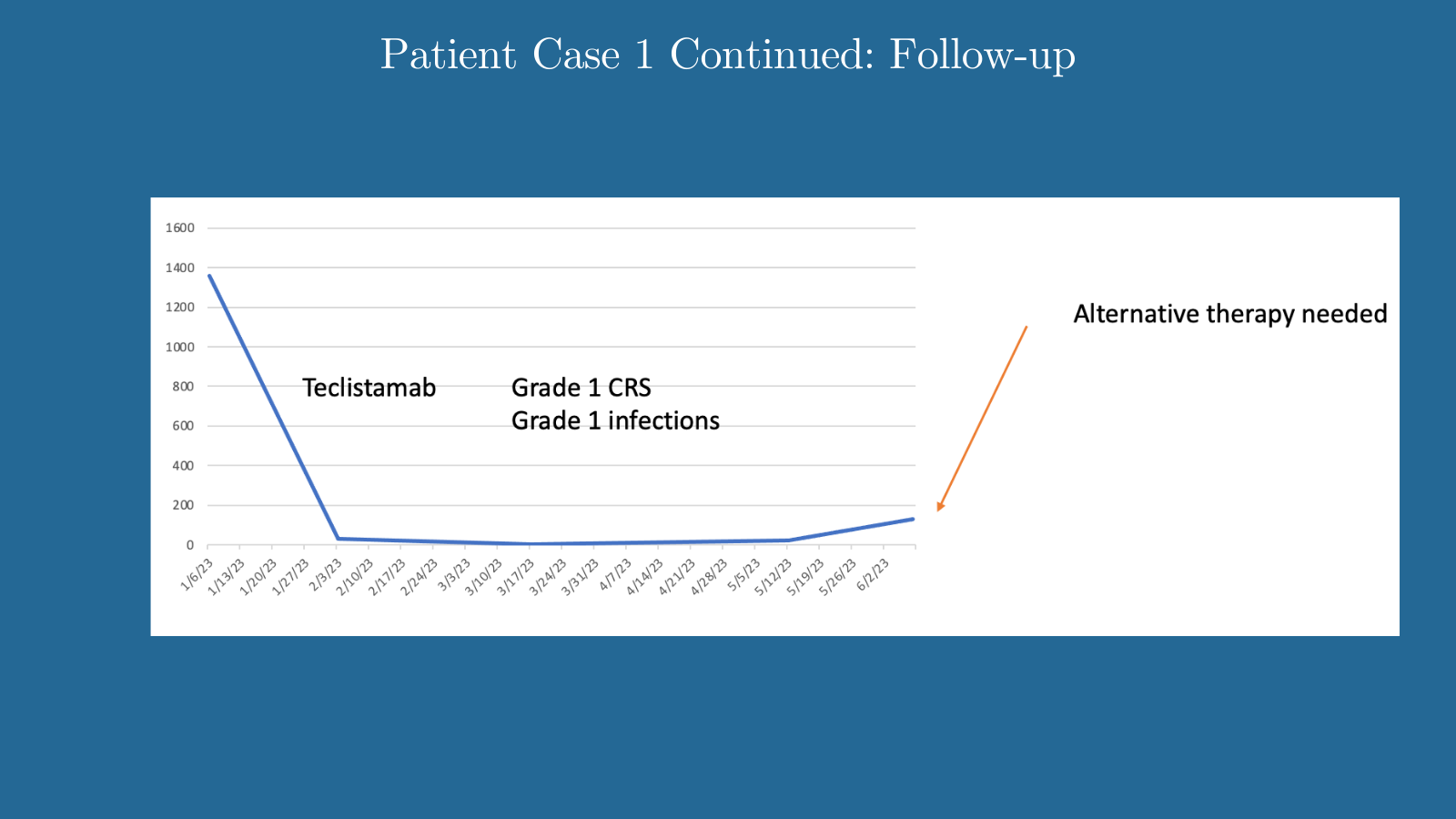
Lipe: There are patients for whom the next line of bispecific therapy may not work as well after they’ve been exposed to some of these bispecific agents. The responses may not be as high, and the durability might not be as high. For patients who are potentially eligible for CAR T therapy and are experiencing long PFS intervals, I don’t want to expose them to these drugs if I don’t have to when I’m ultimately planning to give them CAR T therapy.
Treatment Schedules and Step-Up Dosing
Patient Case 2 Managed by Ajai Chari, MD

Chari: Can you tell us a little bit about the delivery of these bispecific agents in terms of step-up dosing and what you’ve done specifically at your center?
Dhakal: We have good experience with teclistamab now; we have probably treated more than 40 patients with this agent. Fortunately, we have this outpatient program where we’re able to do the teclistamab administration both outpatient and inpatient in eligible patients. In an outpatient setting, patients are seen every day, even in the non-administration day. If they have cytokine release syndrome [CRS] or immune effector cell-associated neurotoxicity syndrome [ICANS], we can monitor them completely in an outpatient [setting] and can administer tocilizumab [Actemra] if needed as an outpatient. We also have a 24-hour clinic which is fully staffed during the day and night. For example, if the patient gets a fever, which is concerning for CRS, they can be seen right away in the 24-hour clinic, have all the infectious workup done, receive tocilizumab, be observed for up to 12 hours, and then be discharged from there before getting back to the clinic the next day. That program has enabled us to give outpatient treatment. Not all patients are going to get outpatient treatment, especially if they don’t have good caregiver support or concerning disease. For example, in my case, my patient was on dialysis, so we couldn’t do outpatient treatment.
Patient Case 2 Continued: Treatment

We have seen that the rate of CRS requiring admission for these patients is low. Those who started treatment as an outpatient can be completely managed as an outpatient. In terms of the talquetamab, we will be doing every-other-week dosing instead of a weekly schedule.
Chari: How else are you handling dosing for these patients with the step-up schedules?
Lipe: I would prefer to do treatment in an all-outpatient setting because one of the challenges we have is bed availability on demand. For most patients, we do this mixture of outpatient and inpatient treatment, which has to do with billing and how we get reimbursed for the drug. At our academic referral center, a lot of our patients come to us to get started on therapy before going back to their providers once they’re stabilized. That can be pretty far away, which has its challenges. At least for our institution, we have this mixture of giving the drug in an outpatient setting, admitting patients for observation, discharging them for 48 hours, giving outpatient treatment again, and then admitting them again. It has become complicated, and it means that we need to have a bed available for certain treatment days. We are also admitting and discharging patients between doses.
Patient Case 2 Continued: Treatment

Matous: We’ve been doing the same thing. However, we are rolling at our outpatient program based on our CAR T outpatient program. We’re going to use wearable [devices to monitor the patients]. We’re also rolling out a study that may be impactful here where patients will receive commercial teclistamab and be pretreated with a single dose of tocilizumab. That’s designed to be a completely outpatient trial, as well.
Key Takeaways
Chari: What are some closing thoughts you have on this topic?
Dhakal: We are in a very great time for our patients with multiple myeloma because we have a lot of good options. And as new data emerge, we just need to know how to sequence these treatments effectively and how to manage the adverse effects effectively in a way that does not impair the quality of life while prolonging the duration of response.
Lipe: One of the take-home messages that I’d like to stress—as someone who lives in a large catchment area that encompasses very rural populations and patients who may be treated very far away—is that this is a unique opportunity to treat patients with these effective therapies, and it’s a unique opportunity for us to engage our colleagues who might not be at an academic center in a new and better way. One of the things we don’t want is access to these great therapies being restricted to the patients, whether that’s due to financial or racial disparities. When we have something so effective, we can’t let that happen. There is a real unique opportunity to partner with our colleagues and our rural partners.
Patient Case 2 Continued

Matous: For relapsed disease in the future, we’re going to see patients with their first relapse who are triple-class refractory or penta-drug exposed. All of us at referral centers need to be able to give these T cell redirecting antibodies. We have to coordinate that in appropriate patients with CAR T-cell therapy. It’s going to involve great communication between referral centers and our colleagues in the community who are seeing the majority of these patients to facilitate getting these amazing treatments to all the patients.
References
- U.S. FDA approved TALVEY (talquetamab-tgvs), a first-in-class bispecific therapy for the treatment of patients with heavily pretreated multiple myeloma. News release. The Janssen Pharmaceuticals. August 10, 2023. Accessed December 15, 2023. https://prn.to/3KwnjyD
- FDA approved teclistamab-cqvy for relapsed or refractory multiple myeloma. News Release. FDA. October 25, 2022. Accessed December 15, 2023. https://bit.ly/3Fgsi4s
- Pfizer’s Elrexfio receives U.S. FDA accelerated approval for relapsed or refractory multiple myeloma. News release. Pfizer. August 14, 2023. Accessed December 15, 2023. https://bit.ly/3DTCRIY
EP: 1.Recent Data in the Management of Early-Relapse Multiple Myeloma
EP: 2.Treatment Decision-Making in Early-Relapse MM
EP: 3.Multiple Myeloma Treatment: Patient Profiles
EP: 4.Recent Data on Bispecific Antibodies in Multiple Myeloma
EP: 5.Dosing Considerations for Bispecific Antibodies in MM
EP: 6.Managing Adverse Effects from Bispecific Antibodies in Multiple Myeloma
EP: 7.MM: Managing Toxicities From BCMA-Targeting Bispecific Antibodies
EP: 8.Toxicities From GPRC5D-Targeting Therapies in Multiple Myeloma
EP: 9.Treatment Decision-Making in Multiple Myeloma
EP: 10.Key Takeaways on the Treatment of Patients With Relapsed/Refractory MM
EP: 11.Bispecific Antibody Dose Schedules in Relapsed/Refractory Multiple Myeloma
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.