Breast Cancer Stem Cells: A New Target for Therapy
The cancer stem cell (CSC) theory was first proposed to explain the fact that only a small proportion of leukemia or solid tumor cells have the capacity to induce growing tumors in immunodeficient mice.[1,2]
Normal adult tissue stem cells awake from a dormant state to grow, differentiate, and regenerate damaged tissue. They also travel in the circulation and colonize distant organs at sites undergoing tissue repair. These same traits are utilized or co-opted by metastatic cancer cells. The cancer stem cell theory proposes that tumors emerge from a subpopulation of cancer cells that possess stem cell properties. This theory has profound implications for therapy. A small number of cancer stem cells may lie dormant following conventional therapy and tumor remission, only to re-emerge and regenerate the entire recurrent cancer. Consequently, it has been proposed that targeting cancer stem cells is the only way to obtain durable cancer treatment responses. Several strategies for targeting cancer stem cells have been proposed. Nevertheless, a number of issues must be investigated and resolved before effective treatments targeting cancer stem cells can enter clinical testing.
The cancer stem cell (CSC) theory was first proposed to explain the fact that only a small proportion of leukemia or solid tumor cells have the capacity to induce growing tumors in immunodeficient mice.[1,2] This tumorigenic subpopulation was found to possess stem cell markers, and to form spheroids in culture. In 1997, Bonnet and Dick isolated a subpopulation of myeloid leukemia cells that express a specific surface marker-CD34-but lack the CD38 marker. These cells were able to initiate leukemia in non-obese diabetic, severe combined immunodeficient (NOD/SCID) mice.[3] In 2003, Al-Hajj and his colleagues demonstrated that only a small subpopulation of CD44+/CD24low cells isolated from human breast cancer tissue were able to develop a tumor in immunodeficient mice.[4]
Following these initial reports, a number of labs have harvested and studied CSCs from virtually every major type of cancer. In vitro, CSCs grow as three-dimensional cellular aggregates, called spheroids, ranging up to 300 microns in diameter. Populations of CSC spheroids can be maintained in vitro after several passages of dissociation.[1] CSCs from different subtypes of cancer have different characteristic surface markers. Breast cancer stem cells are thought to be CD44high and CD24low.[4] In vitro and in animal models, CSCs have been found to be resistant to conventional chemotherapy and are thought to “lie in wait” in a dormant state within the tumor microenvironment, even when the major bulk of non-CSC tumor cells are killed by the therapy.[5]
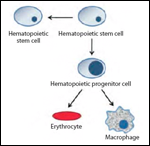
It is possible to monitor CSC division by treatment with PKH26 fluorescent dye prior to transplantation. Studies using this dye have shown that self-renewal in CSCs is frequently driven by symmetric division instead of asymmetric divisions;[6,7] this division leads to the formation of two identical CSCs (or differentiated cells)-unlike division in normal stem cells, in which a single division always results in the formation of a stem cell and a committed cell (Figure 1). CSCs use this mechanism to enrich and regenerate the population within the bulk of the tumor. By dividing in this manner, it is hypothesized that CSCs persist over time to presumably regenerate the tumor in a manner analogous to the regeneration of damaged tissue by normal stem cells.[8,9]
The cellular pathways involved in the regulation of dormancy vs growth within the CSC tumor niche include the Notch,[10] Wnt,[11,12] and Hedgehog[13] pathways. These same pathways are also commonly used by normal stem cells, since they contribute in different ways to “stemness”-for example, by suppressing differentiation or by promoting an adherence-independent state.
Once CSCs have been triggered to emerge, they respond to external stimuli, proliferate, and recapitulate the original histomorphology of the tumor. CSCs, depending on the tissue of origin, express the same markers (eg, CD34 and CD133) and intracellular proteins (eg, ALDH, Nanog, Sox2, and Oct3/4) found in normal stem cells of the same tissue.[14,15] For this reason, many have postulated that CSCs are transformed normal stem cells. Normal stem cells exist in a wide spectrum of differentiated states, ranging from primitive pluripotent cells to partially committed progenitor cells to fully committed and differentiated cells. At any point in this range of commitment states, a neoplastic genetic lesion can occur during cell division. Such an occurrence induces a tumor with a phenotype that is frozen in the differentiated state of the original stem cell or progenitor cell that sustained the genetic carcinogenic hit.[16]
Although CSCs may be resistant to DNA-damaging chemotherapy when they are in a dormant, nonproliferative state, they may also be resistant to standard therapies intrinsically. Glioma CSCs have been shown to be radioresistant; they activate DNA checkpoints and thereby amplify the rate of DNA repair following radiation-induced damage. After radiation therapy, it has been found that glioblastomas are enriched in CD133+ CSCs. This is thought to be a consequence of a higher survival rate of the CSCs compared with that of the bulk of the glioblastoma.[17]
Normal stem cells travel in the circulation, extravasate, intravasate, invade, and colonize normal tissue during regeneration and healing. In addition, normal stem cells induce angiogenesis in healing tissues.[8,18,19] These same physiologic invasion programs are employed by CSCs. Thus, CSCs are thought to be enriched during invasion and metastasis. In fact, the level of stem cell markers in a tumor or a lymph node metastasis has been proposed to correlate with tumor aggressiveness.[20,21]
Tumors are known to be highly heterogeneous. CSCs may contribute to this histologic, cytologic, and morphologic heterogeneity. It has even been suggested that CSCs can contribute to the vascular and stromal elements in the tumor microenvironment.
The CSC theory remains controversial and may not explain many types of cancer; only some types of cancer may be driven by CSCs. Depending on the original cell or cells that are genetically altered during the carcinogenic process, a given tumor may, or may not, be sustained by CSCs.[8,22]
Breast Cancer and Breast Cancer Stem Cells
Breast cancer is the most common form of cancer diagnosed in women worldwide, affecting about 10% of women. Although the rate of mortality as a result of breast cancer has decreased in Western countries due to earlier detection, the incidence of breast cancer has risen by 30% in developed countries in the last decade.[23]
The first evidence of a CSC origin for solid tumors was demonstrated by Al-Hajj et al in 2003 in breast tumors. They isolated a small subpopulation of cells that were CD44+ (alone or in conjunction with ESA [epithelial specific antigen]) and CD24-, and that were able to generate a tumor in NOD/SCID mice. Breast CSCs are propagated in vitro through the formation of anchorage-independent “mammospheres,” a term for mammary cell spheroids. Mammospheres are not composed homogeneously of CD44+/CD24- cells; after dissociation of the spheres and fluorescent-activated cell sorting (FACS) analysis, only a subpopulation of the cells are found to be CD44+/CD24-. Nevertheless, this subpopulation of cells are still able to generate mammospheres in in vitro conditions and to produce tumors in suitable immunodeficient mice.[4]
Breast CSCs (BCSCs) have been examined by many investigators hoping to find a specific marker that can be used for routine identification or to serve as a therapeutic target. Elevated levels of ALDH1 expression give CD44+/CD24- cells higher tumorigenic activity after in vivo assays. Moreover, BCSCs show an enhanced PKH26 dye-retaining capacity, providing an indirect measure of dormancy. PKH26 dye binds irreversibly to cell membranes and it is divided among daughter cells only when a cell undergoes division. The less the cells divide, the less dye is lost. A higher dye content may be a means of identifying quiescent BCSCs.[6]
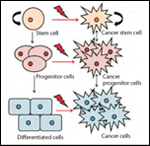
BCSCs can originate from normal breast stem cells within the gland that gives rise to epithelial or myoepithelial cells that line the duct or generate the alveoli.[24] At any stage of cellular differentiation or commitment, a breast stem cell can be subject to a carcinogenic insult (Figure 2). A tumor that forms at this point will retain the differentiated program of the original stem or progenitor cell, and the breast cancer that is established will exhibit a differentiated pattern and a morphology that heralds back to the state of differentiation of the originating breast stem cell or progenitor cell at the time of carcinogenesis.
Breast cancer progenitor cells with stem-like properties, an invasive phenotype, and the propensity to form spheroids have recently been isolated for the first time from human breast premalignant lesions.[19] This finding indicates that the malignant phenotype of breast cancer may be determined very early in the course of the disease and may exist in a dormant state in premalignant lesions, such as ductal carcinoma in situ.
The World Health Organization has classified 30 morphologic types of breast cancer on the basis of histology and molecular alterations. Major categories of breast cancers often used, based on defining molecular subtypes, are luminal type A, luminal type B, the basal type, and the ErbB2-positive type.[25,26] The luminal-type breast cancers are thought to derive from differentiated luminal cells; these are estrogen receptor (ER)-positive and HER2-negative. The basal-type tumors are double negative-that is, negative for both ER and HER2. This type of tumor is thought to derive from luminal progenitor cells, and it is the subtype most commonly associated with BRCA1 mutations. ErbB2-type tumors may be derived from late luminal progenitors that have undergone HER2 gene amplification.
If BCSCs are the cause of breast cancer treatment failure, then why haven’t investigators used BCSC markers to target therapies directly to the BCSCs? The first reason is that stem cell markers found on CSCs are also present in normal tissue stem cells. Thus, it is possible that a therapy that killed cells bearing a stem cell marker would kill normal stem cells as well as CSCs. In addition, markers thought to be expressed on BCSCs may not be specific. The exclusive use of CD44 and CD24 to identify and isolate BCSCs is a controversial topic.[27] The frequency of CD44+/CD24- cells within breast tumors varies significantly depending on the tumor subtype and the histologic stage. CD44+/CD24- cells are generally enriched in basal-type breast cancers as well as in cells that have undergone epithelial-to-mesenchymal transition.[28] In contrast, only about 1% of luminal-type cell populations consist of CD44+/CD24- cells. Moreover, not all CD44+/CD24- cells have the same grade of tumorigenicity if injected into xenografts.[21] Thus, CD44+/CD24- marker levels are not specific for actual tumor-forming cells. Some have suggested that the presence of CD44+/CD24- cells could be more related to the cancer subtype (eg, basal-like), rather than an actual reflection of stem cells within the tumor bulk.[27,29] Since CD44+/CD24- characterization is not sufficient for isolation of the entire BCSC pool, there is a great need for a deeper characterization of the BCSC compartment in order to target these cells for therapy.
Treatment Strategies
Theoretically, we could target CSCs through a combination of strategies that would be specific for the amplified or altered genome of CSCs and spare normal adult stem cells. HER2-amplified, ErbB2+ tumors are currently treated with inhibitors of the epidermal growth factor (EGF) receptor family, including trastuzumab (Herceptin) and lapatinib (Tykerb). However, this treatment would be effective against CSCs only if the HER2 gene were amplified at the level of the stem cell. If a cell with ErbB2 amplification were to emerge after several divisions from a CSC in which there was no original amplification, the cancer stem compartment would not be affected by EGF receptor family drugs. Moreover, CSCs, regardless of HER2 amplification status, may be driven by signaling pathways that are different from those that drive their differentiated progeny. This is why, although drugs that inhibit the EGF receptor family are initially efficacious, there is often the relapse of the tumor.[6]
A second approach is to force the CSCs into a differentiated state, thereby impairing stem characteristics, such as self-renewal. Administration of retinoic acid,[24] or interference with the Notch, Wnt, or Hedgehog pathways that are thought to regulate differentiation,[12] are strategies that have been proposed.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Lapatinib (Tykerb)
Retinoic acid
Trastuzumab (Herceptin)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Another approach is to destabilize the stem cell niche, which is thought to protect the CSCs in a sequestered, non-dividing dormant state. Disregulating the supporting cells in the stem cell niche could theoretically deplete the dormant CSCs.[19] If CSCs are resistant to therapy because they are enriched in drug efflux pumps, then targeting the membrane transporters, such as adenosine triphosphate–binding cassette (ABC) pumps, could potentially sensitize the CSCs to chemotherapy.[1] It has been proposed that reversing the active state of stem cell–related genes, such as Oct 3/4, Sox2, and Nanog, through the use of epigenetic manipulation could knock out the CSC phenotype.[30] CSCs are apoptosis-resistant, it is thought, in order to secure the production of progeny. This resistance could be caused by intracellular mechanisms that lead to a blockade in the apoptotic pathways- for example, an overexpression of anti-apoptotic molecules (Bcl-2, Bcl-XL) or a downregulation of Caspases. These negative regulators of apoptosis are candidate therapeutic targets.[19]
Conclusions
Although the concept of CSCs remains controversial, most investigators acknowledge the presence of a subpopulation of tumor-initiating cells within the tumor bulk. These cells have stem cell characteristics, including self-renewal, pluripotency, and motility. This phenotype is the basis for the name “cancer stem cells.” In breast tumors, as well as in other tumors, CSCs or progenitor cells may constitute a subpopulation that remains following a treatment-induced regression of the bulk of the tumor mass. According to the CSC hypothesis, tumor recurrence results from expansion of the CSC subpopulation in a way that reconstitutes the tumor mass. The CSC hypothesis can explain the prevalence of cancer treatment failure, but presently there are no clinical treatment strategies that directly target CSCs. There is an urgent need to investigate critical biologic questions that may yield strategies for successful and specific targeting of CSCs. These questions include a) when and how CSCs or progenitor cells first originate, b) how CSCs differ from normal adult stem cells, c) which regulatory events are responsible for dormancy or for the emergence of CSCs within a tissue niche, d) how the individual characteristics of tumor-specific CSCs may differ from one patient to the next, and e) how the original genomic characteristics of a cancer stem clone dictate the histology and clinical behavior of the tumor that emerges.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253-70.
2. Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994; 367:645-8.
3. Bhatia M, Wang JC, Kapp U, et al. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997; 94:5320-5.
4. Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983-8.
5. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-84.
6. Cicalese A, Bonizzi G, Pasi CE, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083-95.
7. Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068-74.
8. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-11.
9. Vermeulen L, Sprick MR, Kemper K, et al. Cancer stem cells-old concepts, new insights. Cell Death Differ. 2008;15:947-58.
10. Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169-75.
11. Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86-95.
12. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-50.
13. Zardawi SJ, O’Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24:385-98.
14. Bussolati B, Bruno S, Grange C, et al. Identification of a tumor-initiating stem cell population in human renal carcinomas. Faseb. J 2008;22:3696-705.
15. Tomuleasa C, Soritau O, Rus-Ciuca D, et al. Isolation and characterization of hepatic cancer cells with stem-like properties from hepatocellular carcinoma. J Gastrointestin Liver Dis. 2010;19:61-7.
16. Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274-82.
17. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-60.
18. Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553-7.
19. Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607-11.
20. Brabletz T, Jung A, Spaderna S, et al. Opinion: migrating cancer stem cells-an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744-9.
21. Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59.
22. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755-68.
23. American Cancer Society. Cancer facts and figures 2010. Atlanta: American Cancer Society; 2010.
24. Gudjonsson T, Ronnov-Jessen L, Villadsen R, et al. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115(Pt 1):39-50.
25. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-52.
26. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869-74.
27. Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat. 2009;118:241-54.
28. Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI. The CD44+/CD24- phenotype relates to ‘triple-negative’ state and unfavorable prognosis in breast cancer patients. Med Oncol. 2010; [Epub ahead of print]
29. Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259-73.
30. Ting AH, McGarvey KM, Baylin SB. The cancer epigenome-components and functional correlates. Genes Dev. 2006;20:3215-31.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.