Campath Active in Refractory B-CLL With p53 Mutation
ORLANDO-Alemtuzumab (Campath-1H) can induce responses in patients with refractory B-cell chronic lymphocytic leukemia (B-CLL) who have the 17p-/p53 genetic mutation, which is usually characterized by a dismal outcome, Stephan Stilgenbauer, MD, of the Department of Internal Medicine III, University of Ulm, Germany, reported at the 43rd Annual Meeting of the American Society of Hematology (abstract 3211). The researchers used alemtuzumab to treat 11 patients with B-CLL, as well as 4 with T-prolymphocytic leukemia (T-PLL) and 1 with Sézary syndrome.
ORLANDOAlemtuzumab (Campath-1H) can induce responses in patients with refractory B-cell chronic lymphocytic leukemia (B-CLL) who have the 17p-/p53 genetic mutation, which is usually characterized by a dismal outcome, Stephan Stilgenbauer, MD, of the Department of Internal Medicine III, University of Ulm, Germany, reported at the 43rd Annual Meeting of the American Society of Hematology (abstract 3211). The researchers used alemtuzumab to treat 11 patients with B-CLL, as well as 4 with T-prolymphocytic leukemia (T-PLL) and 1 with Sézary syndrome.
The small, single-institution study was based on a larger study that produced an overall response rate of 33% among 93 B-CLL patients who were refractory to fludarabine (Fludara) and received alemtuzumab. The previous study also raised questions about possible associations between the drug and genetic factors such as 17p-.
The new study involved 16 patients. All 11 B-CLL patients were fludarabine refractory. The four T-PLL patients and one with Sézary syndrome were also chemotherapy refractory.
After initial alemtuzumab dose escalation from 3 to 10 to 30 mg, all patients received three doses at 30 mg IV per week for a maximum of 12 weeks. Premedication was with antihistamines and acetaminophen, with additional drugs used for infusion-related side effects and infection control.
One patient with B-CLL received alemtuzumab as in vivo purging as part of the high-dose regimen given before autologous stem cell transplantation; this was due to a poor graft with high B-cell contamination.
Responses and Reactions
The B-CLL and T-PLL groups each had one complete responder. In addition, there were four partial responders in the B-CLL group and two in the T-PLL group, Dr. Stilgenbauer reported. Responses were rapid in blood and bone marrow but delayed for splenomegaly and lymphadenopathy.
Grade 3-4 infections occurred in five patients and acute coronary syndromes in two patients. The two cases of treatment-related mortality were from sepsis and pneumonia.
One T-PLL patient experienced intolerable infusion-related reactions, but tolerated subcutaneous alemtuzumab without premedication and went on to achieve a partial remission. The two T-PLL partial responders went on to allogeneic stem cell transplant; one subsequently died of aspergillus pneumonia. The B-CLL patient receiving alemtuzumab as in vivo purging achieved a partial response.
Genetic Risk Factors
Several genetic risk factors were evaluated, including mutations of the p53 gene and VH genes. Among the refractory B-CLL patients, four had unmutated VHthree of these had a response, including one complete response.
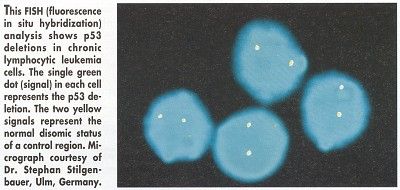
Two B-CLL patients had the 17p- high-risk genetic aberration and mutations in the remaining p53 allele. "In CLL, 17p- is associated with resistance to chemotherapy," Dr. Stilgenbauer noted. "That has been shown for alkylating agents as well as purine analogs."
In both of these patients, treatment with alemtuzumab achieved rapid clearing of lymphocytes. One patient had to stop treatment because of pneumonia and subsequently died. The other patient achieved a complete remission that, at the time of the ASH presentation, exceeded 12 months.
Dr. Stilgenbauer and his colleagues concluded that achieving complete response in B-CLL with inactivation of p53 appears to indicate a p53-independent mechanism of action, and makes alemtuzumab an option for B-CLL patients with the 17p-/p53 genetic mutation.