Cancer Management Chapter 9: Esophageal cancer
Although still relatively uncommon in Western countries, esophageal cancer is fatal in the vast majority of cases. In the United States, an estimated 16,470 new cases will be diagnosed in the year 2009, and 14,530 deaths will result from the disease. This high percentage of deaths rivals that of pancreatic cancer and is more than four times that of rectal cancer.
Although still relatively uncommon in Western countries, esophageal cancer is fatal in the vast majority of cases. In the United States, an estimated 16,470 new cases will be diagnosed in the year 2009, and 14,530 deaths will result from the disease. This high percentage of deaths rivals that of pancreatic cancer and is more than four times that of rectal cancer.
The esophagus extends from the cricopharyngeal sphincter to the gastroesophageal (GE) junction and is commonly divided into the cervical, upper to mid-thoracic, and thoracic portions. This can be important, as histology and optimal treatment approaches may vary considerably based on the site of the cancer. It may not be possible to determine the site of origin if the cancer involves the GE junction itself.
Epidemiology
Gender Esophageal cancer is seven times more common and slightly more lethal in men than in women.
Age Adenocarcinoma of the esophagus (now more common in the United States than the squamous cell type) has a median age at diagnosis of 69 years. The incidence of squamous cell cancer of the esophagus increases with age as well and peaks in the seventh decade of life.
Race The incidence of squamous cell esophageal cancer is three times higher in blacks than in whites, whereas adenocarcinomas are more common in white men.
Geography Evidence of an association between environment and diet and esophageal cancer comes from the profound differences in incidence observed in various parts of the world. Esophageal cancer occurs at a rate 20 to 30 times higher in China than in the United States. An esophageal “cancer belt” extends from northeast China to the Middle East.
Survival Although the overall outlook for patients diagnosed with esophageal cancer has improved in the past 30 years, most patients still present with advanced disease, and their survival remains poor. One-third to one-half of patients treated with either chemoradiation therapy or chemoradiation therapy plus surgery are alive at 2 years, without recurrence of esophageal cancer.
Disease site The rate of cancer of the distal esophagus is about equal to that of the more proximal two-thirds. In general, squamous cell carcinoma is found in the body of the esophagus, whereas adenocarcinoma predominates in lesions closer to the GE junction.
Etiology and risk factors
Cigarettes and alcohol Squamous cell carcinomas of the esophagus have been associated with cigarette smoking and/or excessive alcohol intake. Furthermore, cigarette smoking and alcohol appear to act synergistically, producing high relative risks in heavy users of tobacco and alcohol. Esophageal adenocarcinoma is increased twofold in smokers.
Diet High-fat, low-protein, and low-calorie diets have been shown to increase the risk of esophageal cancer. Exposure to nitrosamines has been proposed as a factor in the development of both squamous cell carcinoma and adenocarcinoma of the esophagus.
Barrett's esophagus and other factors Gastroesophageal reflux disease (GERD) and Barrett's esophagus (adenomatous metaplasia of the distal esophagus) have been linked to adenocarcinoma of the esophagus. Tylosis, Plummer-Vinson syndrome, history of head and neck cancer, and achalasia have also been associated with a higher-than-normal risk of developing squamous cell cancer of the esophagus.
Signs and symptom
Because symptoms do not alert the patient until the disease is advanced, few esophageal cancers are diagnosed at an early stage.
Dysphagia The most common presenting complaint is dysphagia, which generally is not noted until the esophageal lumen is narrowed to one-half to one-third of normal, due to its elasticity.
Weight loss is common and has a significant role in prognosis (> 10% of total body weight as poor prognosis).
Cough that is induced by swallowing is suggestive of local extension into the trachea with resultant tracheoesophageal fistula.
Odynophagia and pain Pain with swallowing (odynophagia) is an ominous sign. Patients who describe pain radiating to the back may well have extra-esophageal spread. Supraclavicular or cervical nodal metastases may be appreciated on examination.
Hoarseness may be a sign of recurrent laryngeal nerve involvement due to extraesophageal spread.
Metastatic disease may present as malignant pleural effusion or ascites. Bone metastasis can be identified by pain involving the affected site or by associated hypercalcemia. The most common metastatic sites are retroperitoneal or celiac lymph nodes.
The American College of Surgeons conducted a study utilizing its national cancer database to assess the presentation, stage distribution, and treatment of patients diagnosed with esophageal cancer between 1994 and 1997 (n = 5,044). The most common presenting symptoms were dysphagia (74.0%), weight loss (57.3%), reflux (20.5%), odynophagia (16.6%), and dyspnea (12.1%). The American College of Surgeons Database finds 50% of patients present with tumors in the lower third of the esophagus; 42% have adenocarcinoma histology, and 52% have squamous histology. Barrett's esophagus was found in 39% of those patients with adenocarcinoma. Patients undergoing initial surgical resection had the following stage distribution: stages I (13.3%), II (34.7%), III (35.7%), and IV (12.3%).
Diagnosis
In Western countries, the diagnosis of esophageal cancer is generally made by endoscopic biopsy of the esophagus. In the Far East, cytologic evaluation is frequently utilized.
Endoscopic ultrasonography (EUS) is extremely accurate (> 90%) in establishing the depth of tumor invasion (T stage) but less accurate (70%–80%) in determining nodal involvement (N stage) unless combined with fine-needle aspiration (FNA) of the involved nodes (93% accuracy) when nodes greater than 5 mm are biopsied. The addition of FNA increases the sensitivity from 63% to 93% and the specificity from 81% to 100%. EUS is not reliable in determining the extent of response to neoadjuvant treatment.
Endoscopy and barium x-rays Endoscopy allows for direct visualization of abnormalities and directed biopsies. Barium x-rays are less invasive and provide a good assessment of the extent of esophageal disease.
Bronchoscopy should be performed to detect tracheal invasion in all cases of esophageal cancer except adenocarcinoma of the distal third of the esophagus.
CT scan Once a diagnosis has been established and careful physical examination and routine blood tests have been performed, a CT scan of the chest, abdomen, and pelvis should be obtained to help assess tumor extent, nodal involvement, and metastatic disease.
PET A prospective trial designed to evaluate the utility of PET vs CT and EUS was performed by obtaining these studies in 48 consecutive patients prior to esophagectomy. PET achieved a 57% sensitivity, a 97% specificity, and an 86% accuracy compared with CT, which was 99% sensitive, 18% specific, and 78% accurate. In terms of nodal staging, PET was correct in 83% of cases, as compared with 60% of cases for CT and 58% for EUS (P = .006). This analysis suggests the improved accuracy of PET in the staging work up of patients with esophageal cancer.
Numerous studies report the accuracy of PET scanning in determining the presence of metastatic disease, with sensitivity approaching 90% and specificity over 90%.
As PET becomes more widely available, its use will probably become an important part of the preoperative evaluation of these patients. In a prospective trial of 39 patients with esophageal cancer, PET detected additional sites of metastatic disease at the initial evaluation when compared with conventional imaging. After induction therapy, PET did not add to the estimation of locoregional resectability and did not detect new distant metastases. However, this study suggested that changes in ([18fluorodeoxyglucose]) FDG-PET following induction therapy may predict disease-free and overall survival after induction therapy and resection in patients with esophageal cancer. A large prospective national trial will evaluate the use of PET in the treatment of esophageal cancer.
Bone scan A bone scan should be obtained if the patient has bone pain or an elevated alkaline phosphatase level.
Thoracoscopy/laparoscopy Investigators have begun to examine the role of surgical staging prior to definitive therapy. These procedures are designed to allow pathologic review of regional lymph nodes and the accurate assessment of extraesophageal tumor spread by direct visualization. A multi-institution trial (CALGB 9380) found these procedures to be feasible in over 70% of patients; they resulted in the upstaging of patients in 38% of cases reviewed. Further investigations need to be completed to determine the appropriate use of these tools in treatment algorithms for patients with esophageal cancer.
Warning Staging studies should be performed in a sequential manner. Invasive, lower yield, and less accurate studies and procedures should only be undertaken if management would change on the basis of specific findings.
Screening and surveillance
High-risk patients
Adenocarcinoma The role of screening patients with GERD and surveillance of patients with Barrett's esophagus by upper GI endoscopy remains under investigation. In 833 patients studied by endoscopy, there was a 13% incidence of intestinal metaplasia (Barrett's esophagus). Dysplasia or cancer was seen in 31% of patients with long-segment Barrett's esophagus, in 10% of short-segment Barrett's esophagus, and in 6% of GE-junction intestinal metaplasia.
Squamous cell carcinoma Mass screening in the high-risk areas of China and Japan is considered appropriate.
Pathology
Adenocarcinoma The incidence of esophageal adenocarcinoma involving the GE junction has risen 4% to 10% per year since 1976 in the United States and Europe. As a result, adenocarcinoma is now the predominant histologic subtype of esophageal cancer. The distal one-third of the esophagus is the site of origin of most adenocarcinomas.
Squamous cell carcinomas occur most often in the proximal two-thirds of the esophagus. Squamous cell carcinoma is still the most prevalent histologic subtype worldwide.
Other tumor types Other, less frequently seen histologic subtypes include mucoepidermoid carcinoma, small-cell carcinoma, sarcoma, adenoid cystic leiomyosarcoma, and primary lymphoma of the esophagus. Occasionally, metastatic disease from another site may present as a mass in the esophagus or a mass pressing on the esophagus.
Metastatic spread The most common sites of metastatic disease are the regional lymph nodes, lungs, liver, bone, adrenal glands, and diaphragm. Adenocarcinoma can also metastasize to the brain.
Staging and prognosis
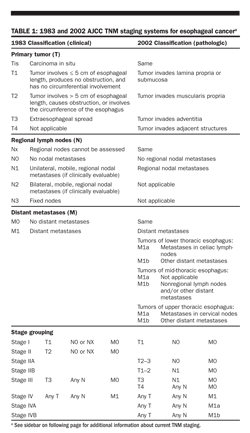
Based on data demonstrating that the depth of penetration has important prognostic significance, the American Joint Committee on Cancer (AJCC) TNM staging system for esophageal cancer was changed from a clinical one (1983) to a pathologic one in 2002. Both the clinical and pathologic staging systems are shown in Table 1, as patients may be cured without an operation. Although pathologic information obtained from an esophagectomy specimen is of prognostic importance, postoperative therapy to improve prognosis has not been rigorously tested. Moreover, recurrence rates for stages I (30%) and II (70%) cancers suggest early systemic spread undetected by current noninvasive staging.
As this Handbook went to press the American Joint Committee on Cancer (AJCC) published an updated edition of the AJCC Cancer Staging Manual. Changes were made to the staging system for esophageal cancer. These included the following: Redefinition of Tis, N, and M; Subclassification of T4 and N; separate stage groupings for squamous cell carcinoma and adenocarcinoma; reassignment of stage groupings using T, N, M, and G classifications. For additional information see Edge SB et al: AJCC Cancer Staging Manual, 7th edition, chapter 10. Springer-Verlag, New York, 2010. Watch also for updates online at
www.cancernetwork.com/cancer-management
.
Pathologic information obtained from an esophagectomy specimen is of significant prognostic importance. Immunohistochemical analysis of the initial biopsy specimen may also have prognostic relevance. Clinical staging has been shown to be of prognostic importance, particularly in patients managed with primary radiotherapy or chemoradiation therapy.
Histology and grade Neither histology nor grade has been shown to be of prognostic importance in esophageal carcinoma.
Other prognostic factors Patient age, performance status, and degree of weight loss are of prognostic importance. The prognostic implications of tumor-suppressor genes and oncogenes are an area of active investigation.
Treatment
Treatment options for the various disease stages are given in Table 2, along with 5-year survival rates.
Treatment of localized disease
Only 40% to 60% of patients with esophageal cancer present with clinically localized disease. The National Comprehensive Cancer Network (NCCN) guidelines state that patients with clinically
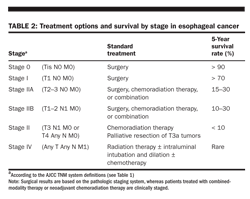
localized disease may be treated with resection or chemotherapy plus irradiation (Tables 3 and 4). The overall 5-year survival rates for either surgery alone or combined chemotherapy and irradiation appear equivalent.
Chemoradiation therapy as primary management of localized or locoregionally confined esophageal cancer has been shown to be superior to irradiation alone. A series of randomized trials have demonstrated that adjuvant postoperative chemoradiation therapy does not offer a survival advantage to patients with esophageal cancer. Adequate patient selection, tumor staging, and treatment standardization will be required before the optimal therapeutic modalities in these patients will be determined.

SurgeryPreoperative medical evaluation helps determine the patient's risk of developing postoperative complications and mortality. In addition to the staging and nutritional status, it should include an evaluation of the pulmonary, cardiac, renal, and hepatic functions.
Extent of surgical resection The extent of resection depends on the location of the primary tumor, histology of the tumor, and nature of the procedure (palliative vs curative). A retrospective study has reported that superficial mucosal lesions may be treated via endoscopic mucosal resection, but those patients with submucosal invasion require esophagectomy. For tumors of the intrathoracic esophagus (squamous cell carcinomas) and tumors with extensive Barrett's esophagus (adenocarcinomas), it is necessary to perform a total esophagectomy with cervical anastomosis to achieve a complete resection. For distal lesions of the abdominal esophagus (adenocarcinomas) and cardia, it is often possible to perform an intrathoracic esophageal anastomosis above the azygos vein, although many surgeons would prefer to perform a total esophagectomy.
The resected esophagus may be replaced with tubularized stomach in patients with tumors of the intrathoracic esophagus or with a colon interposition in patients with tumors involving the proximal stomach, because such involvement makes this organ unsuitable for esophageal reconstruction. The esophageal replacement is usually brought up through the posterior mediastinum, although the retrosternal route is often used in palliative procedures.
Patient selection The indications for esophagectomy in esophageal cancer vary from center to center within the United States.
Clearly, patients with distant metastases, evidence of nodal metastases in more than one nodal basin, or tumor extension outside the esophagus (airway, mediastinum, vocal cord paralysis) are candidates for palliative therapy. Patients with disease limited to the esophagus and no evidence of nodal metastases (stages I and IIA) may be treated with esophagectomy, although these patients can also be considered for definitive treatment with chemoradiation therapy.
Method of resection Considerable controversy also exists among surgeons regarding the method of resection. To date, two randomized studies have compared transhiatal esophagectomy (without thoracotomy) with the Ivor-Lewis (transthoracic) esophagectomy (with thoracotomy). These studies failed to show differences between the two procedures with regard to operative morbidity and mortality. In a randomized trial of 220 patients treated with either a transthoracic or transhiatal esophageal resection, there was a trend toward an improvement in 5-year survival. A meta-analysis failed to show differences in 5-year survival rates. Over the past 5 years, successful attempts have been made to use minimally invasive approaches to esophageal cancer with thoracoscopy and laparoscopy. Although those studies have shown a decrease in morbidity and the minimally invasive approach appears to be oncologically sound from the point of view of resection margins, the number of nodes resected is still not comparable to that of the standard transthoracic approach.
The need for pyloric drainage (pyloroplasty) following esophagectomy is another area of debate. A meta-analysis of nine randomized trials that included 553 patients showed a trend favoring pyloric drainage in improving gastric emptying and nutritional status, whereas bile reflux was better in the nondrainage group. The gastric emptying time evaluated by scintigraphy was twice as long in the nondrainage group as in the pyloric drainage groups.
Lymphadenectomy Considerable controversy exists regarding the need for radical lymphadenectomy in esophageal disease. Much of the controversy is due to the fact that different diseases are being compared.
Japanese series include mostly patients with squamous cell carcinomas of the intrathoracic esophagus, with 80% of the tumors located in the proximal and middle sections of the esophagus. Americans report combined series, with at least 40% to 50% of patients with adenocarcinomas of the distal esophagus. Skinner and DeMeester favor en bloc esophagectomy with radical (mediastinal and abdominal) lymphadenectomy, based on 5-year survival rates of 40% to 50% in patients with stage II disease, as compared with rates of 14% to 22% in historic controls.
In a retrospective study, Akiyama found a 28% incidence of cervical node metastases in patients with squamous cell carcinomas located in the middle and distal portions of the esophagus, as opposed to 46% in those with tumors of the proximal third. Overall survival at 5 years was significantly better in patients who underwent extended lymphadenectomy (three fields) than in those who had conventional lymphadenectomy (two fields); this finding was true in patients with negative nodes (84% and 55%, respectively) and in those with positive nodes (43% and 28%, respectively). Extended lymphadenectomy afforded no survival advantage in patients with tumors in the distal third of the esophagus.
In a study of 1,000 patients with esophagogastric junction adenocarcinomas, the tumors were classified according to the location of the center of the tumor mass in adenocarcinomas of the distal esophagus, cardia, and subcardia. The tumors located in the cardia and subcardia regions spread primarily to the paragastric and left gastric vessel nodes and did not benefit from extended esophagectomy. Kato et al have studied the use of sentinel node mapping to improve the sensitivity of lymphadenectomy.
Preoperative chemotherapy
The frequency of metastatic disease as the cause of death in patients with esophageal cancer has resulted in exploration of the early application of systemic therapy for esophageal cancer. The first of the two large studies was intergroup study 113. A total of 440 patients were treated with surgical resection alone or preceded by 3 cycles of cisplatin and fluorouracil (5-FU). Objective responses were reported in only 19% of patients receiving chemotherapy. No difference in resectability, operative mortality, median survival (14.9 months with chemotherapy vs 16.1 months with surgery alone), or 2-year survival (35% vs 37%) was reported.
However, the Medical Research Council evaluated 802 patients with resectable esophageal cancer in a similar study. Patients randomized to receive chemotherapy were administered 2 cycles of cisplatin (80 mg/m2) and 5-FU (1 g/m2/d as a continuous infusion for 4 days). Microscopically complete resection was performed more frequently in patients receiving chemotherapy, with no difference found in postoperative complications or mortality. Moreover, patients receiving neoadjuvant chemotherapy had significantly longer median (16.8 months vs 13.3 months) and 2-year survival (43% vs 34%) than patients treated with surgery alone. With a median follow up of 6 years, the updated results of this study continued to demonstrate a significant difference in overall survival at 5 years: 23% in patients who received preoperative chemotherapy, vs 17%, and this benefit was present in both histologies. The reasons for the differences in the outcomes are unclear but may be related to the chemotherapy regimen and schedule employed in the intergroup study, patient population, or study design. As a result, the role of neoadjuvant chemotherapy remains in question but is promising, especially with the potentially more efficacious newer generation of chemotherapy agents.
Polee et al have evaluated a biweekly combination of cisplatin and paclitaxel in this setting in a phase II study, with promising results. Objective responses occurred in 59% of 49 patients. No patients had progressive disease. Although 71% of patients had severe neutropenia, it was often asymptomatic. Forty-seven patients underwent resection subsequently. Complete pathologic responses occurred in 14% of patients. The median survival of patients in this study was 20 months, but it was 32 months in patients who had disease responsive to chemotherapy. The 3-year survival rate was 32%.
Given the uncertainty about the efficacy of preoperative chemotherapy and chemoradiation therapy, some investigators have administered preoperative chemotherapy, followed by chemoradiation therapy, then surgery. The true utility of this approach will need to be defined by randomized studies, but clearly, it is feasible, without a significant increase in toxicity or operative morbidity. Interestingly, these reports have also demonstrated that most patients had significant improvement or resolution of dysphagia with the induction chemotherapy alone.
Adjuvant/postoperative chemotherapy
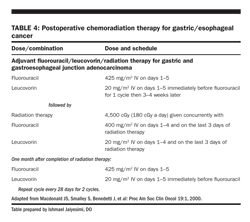
As the most common source of treatment failure in patients with esophageal cancer who have undergone surgical resection, postoperative chemotherapy has also undergone limited investigation. The JCOG Group has compared preoperative and postoperative chemotherapy in 330 patients with stage II or III esophageal squamous cell carcinomas. Patients received 2 courses of either preoperative or postoperative chemotherapy with cisplatin/5-FU. The patients who received preoperative chemotherapy demonstrated a significant improvement in progression–free survival (2.9 years, vs 2.0 years). Thus, the investigators determined that preoperative therapy would be the new standard therapy.
Radiotherapy
Although radiotherapy alone is inferior to chemoradiation therapy in the management of locoregionally confined esophageal cancer, it may offer palliation to patients with advanced local disease too frail for chemotherapy.
Preoperative radiotherapy has been shown to be of little value in converting unresectable cancers into resectable ones or in improving survival. However, it decreases the incidence of locoregional tumor recurrence.
Postoperative radiotherapy (usually to 50 or 60 Gy) can decrease locoregional failure following curative resection but has no effect on survival.
Brachytherapy Intraluminal isotope radiotherapy (intracavitary brachytherapy) allows high doses of radiation to be delivered to a small volume of tissue. Retrospective studies suggest that a brachytherapy boost may result in improved rates of local tumor control and survival over external-beam radiotherapy alone. This technique can be associated with a high rate of morbidity if not used carefully.
A multi-institution prospective trial was conducted by the Radiation Therapy Oncology Group (RTOG) to determine the feasibility and toxicity of chemotherapy, external-beam irradiation, and esophageal brachytherapy in potentially curable patients with esophageal cancer. Nearly 70% of patients were able to complete external-beam irradiation, brachytherapy, and at least 2 cycles of 5-FU/cisplatin. The median survival was 11 months, and the 1-year survival was 49%. Because of the 12% incidence of fistula formation, the investigators urged caution in the routine application of brachytherapy as part of a definitive treatment plan.
Stahl et al updated an earlier publication of a randomized trial comparing preoperative chemotherapy with chemoradiotherapy in 126 patients with T3–4NX adenocarcinoma of the lower esophagus/gastric cardia. Again, pathologic complete response was higher (15.6% vs 2%) and 3–year survival was trending toward improvement (47% vs 28%) in those patients receiving preoperative chemoradiotherapy
(Stahl M, l: J Clin Oncol 27:851–856, 2009)
.
Chemoradiation therapy
Preoperative chemoradiation therapy Initial trials of preoperative chemoradiation therapy reported unacceptably high operative mortality (~26%). Subsequent trials reported operative mortality of 4% to 11%, median survival as long as 29 months, and 5-year survival rates as high as 34%. In general, 25% to 30% of patients have no residual tumor in the resected specimen, and this group tends to have a higher survival rate than those who have a residual tumor discovered by the pathologist.
The superiority of preoperative chemoradiation therapy over surgery alone in esophageal adenocarcinoma has been investigated in several prospective trials. The first trial included 113 patients with adenocarcinoma of the esophagus. These patients were randomized to receive either preoperative chemoradiation therapy (2 courses of 5-FU and cisplatin given concurrently with 40 Gy of radiotherapy in 15 fractions) or surgery alone. Median survival was statistically superior in the combined-modality arm than in the surgery-alone arm (16 months vs 11 months). Rates of 3-year survival again statistically favored the combined-modality arm (32% vs 6%). Although toxicity was not severe, the short survival in the surgery control arm has minimized the impact of these results in the United States.
Another trial performed at the University of Michigan enrolled 100 patients and randomized them to undergo surgery alone or preoperative chemoradiation therapy (cisplatin, 20 mg/m2/d), 5-FU (300 mg2/d), and vinblastine (1 mg/m2/d) and radiotherapy (45 Gy/1.5 Gy bid). With a median follow-up of 8.2 years, the 3-year survival was 16% (surgery alone) vs 30% (induction chemoradiation therapy). This difference did not reach statistical significance, as the study was designed to detect a relatively large increase in median survival from 1 to 2.2 years.
An Australian study of 257 patients by Burmeister et al also found no overall or progression-free survival advantage for those patients randomized to receive preoperative chemoradiation therapy vs surgery alone.
A meta-analysis of randomized trials comparing neoadjuvant chemoradiation therapy followed by surgery with surgery alone found that neoadjuvant concurrent chemoradiation therapy improved 3-year survival (odds ratio, 0.66) compared with surgery alone, with a nonsignificant trend toward increased treatment mortality with neoadjuvant chemoradiation.
Newer chemotherapy agents are active and may improve outcome over these older trials. A phase II trial of 129 patients employed paclitaxel/carboplatin/5-FU with 45 Gy of radiation therapy followed by esophagectomy. A pathologic complete response was seen in 38% of patients, with a median survival of 22 months and a 3-year survival of 41%.
Another phase II trial from the University of Michigan administered paclitaxel/cisplatin with 45 Gy of radiation therapy twice daily (1.5 Gy bid). In this study, 19% of patients exhibited a pathologic complete response, with a 24-month median survival and a 3-year survival of 34%. A phase II trial from Memorial Sloan-Kettering Cancer Center combined cisplatin and irinotecan with 50.4 Gy of radiation therapy followed by surgery. Twenty-five percent of patients had a pathologic complete response. Ongoing studies by the RTOG are employing these newer chemotherapy agents.
Primary chemoradiation therapy Patients with locally advanced esophageal cancer (T1–4 N0–1 M0) may be cured with definitive chemoradiation therapy. Randomized trials have demonstrated a survival advantage for chemoradiation therapy over radiotherapy alone in the treatment of esophageal cancer. In an RTOG randomized trial involving 129 patients with esophageal cancer, irradiation (50 Gy) with concurrent cisplatin and 5-FU provided a significant survival advantage (27% vs 0% at 5 years) and improved local tumor control over radiation therapy alone (64 Gy). Median survival also was significantly better in the combined-therapy arm than in the irradiation arm (14.1 months vs 9.3 months).
Bedenne et al presented a randomized trial of preoperative chemoradiation therapy vs chemoradiation therapy alone, in which there was no difference in median or 2-year overall survival rates. A randomized intergroup trial was designed to investigate the role of high-dose irradiation in conjunction with systemic therapy. This study compared doses of 50.4 Gy with 64.8 Gy. Both treatment arms of the study administered concurrent 5-FU and cisplatin. This trial was stopped after an interim analysis revealed no statistically significant difference in survival between the two groups. The authors concluded that higher dose radiation therapy did not offer any survival benefit over the 50.4-Gy dose.
Patient selection Patients with disease involving the mid to proximal esophagus are excellent candidates for definitive chemoradiation therapy because resection in this area can be associated with greater morbidity than resection of more distal tumors.
Most of the trials demonstrating the efficacy of chemoradiation therapy have had a high proportion of patients with squamous cell cancers. Chemoradiation therapy has thus become standard treatment of locoregionally confined squamous cell cancer of the esophagus. It is essential that chemotherapy be given concurrently with irradiation when this approach is chosen as primary treatment for esophageal cancer. A typical regimen is 50 to 60 Gy over 5 to 6 weeks, with cisplatin (75 mg/m2) and 5-FU (1 g/m2/24 hours for 4 days) on weeks 1, 5, 8, and 11.
The literature also supports offering primary surgery, preoperative chemoradiation therapy, or primary chemoradiation therapy with surgical salvage if necessary to patients with adenocarcinoma. Entering these patients on protocols will allow us to further define standard treatment.
Sequential preoperative chemotherapy and radiation therapy Only modest benefits have been found with preoperative chemoradiation therapy to date, with systemic failure continuing to be an important problem. Thus, sequential therapy with chemotherapy followed by chemoradiation therapy has been explored.
Ajani et al reported a series of 43 patients who received 12 weeks of cisplatin and irinotecan followed by weekly paclitaxel with infusional 5-FU and concurrent radiation therapy (4,500 cGy) and then esophagectomy. Therapy was well tolerated, with no deaths from chemotherapy or chemoradiation therapy, and an operative mortality rate of 5%. Cisplatin and irinotecan induced responses in 37% of patients, and 91% of patients underwent complete resection. Pathologic complete responses occurred in 26% of patients, and some tumor shrinkage was noted in 63% of patients. With a median follow-up of more than 30 months, the median progression-free survival was 10.2 months, the median survival was 22.1 months, and the 2-year survival was 42%. The patients who had a pathologic response to therapy had significantly better outcomes than the rest of the study population. However, systemic recurrences remained a prominent cause of failure, with five patients experiencing recurrence first in the brain and an additional five patients, in the liver.
Esophagectomy following induction chemotherapy and chemoradiation therapy Controversy exists regarding the need for esophagectomy following chemoradiation therapy. Although previously described studies randomized patients to receive surgery with or without preoperative chemoradiation therapy, Stahl et al randomized patients to receive chemoradiation therapy with or without surgery. Also, all 172 patients in the study underwent initial induction chemotherapy (bolus 5-FU, leucovorin, etoposide, and cisplatin for 3 cycles). Those randomized to receive preoperative chemoradiation therapy received cisplatin/etoposide with 40 Gy of radiation, followed by surgery 3 to 4 weeks later. Those randomized to receive definitive chemoradiation therapy received cisplatin/etoposide with 65 Gy of radiation.
After a 6-year median follow-up, the local progression-free survival favored the group undergoing surgery (64% vs 41%). However, the treatment-related mortality was higher in those patients undergoing surgery (13% vs 4%), and so overall survival was statistically equivalent (at 3 years, 31% vs 24%). Since induction chemotherapy was used in all patients, these results should not be extrapolated to indicate the value of esophagectomy following chemoradiotherapy alone.
The incidence of residual disease in patients who have a complete clinical response to chemoradiation therapy is 40% to 50%, and those patients who have a pathologic complete response to chemoradiation therapy have the best survival rates with surgery.
Treatment in elderly patients Since more patients are being diagnosed with esophageal cancer at older ages, research is ongoing as how best to treat elderly patients. Retrospective studies from Nallapareddy et al have found chemoradiaton therapy is tolerable in elderly patients, whereas Rice et al have found a trimodality approach of chemoradiation therapy followed by surgery is also tolerable in the elderly. Close monitoring for toxicities such as dehydration, nutritional concerns, anemia, and postoperative arrhythmia was recommended in these two studies.
Treatment of advanced disease
The goal of esophageal cancer treatment is generally palliative for patients with bulky or extensive retroperitoneal lymph nodes or distant metastatic disease. Therapeutic approaches should temper treatment-related morbidity with the overall dismal outlook.
Local treatment In patients with a good performance status, the combination of 5-FU/mitomycin, or 5-FU/cisplatin, and radiotherapy (50 Gy) results in a median survival of 7 months to 9 months. This regimen usually renders patients free of dysphagia until death.
Photodynamic therapy (PDT) Porfimer (Photofrin) and an argon-pumped dye laser can provide effective palliation of dysphagia in patients with esophageal cancer. A prospective, randomized multicenter trial comparing PDT with neodymium/yttrium-aluminum-garnet (Nd:YAG) laser therapy in 236 patients with advanced esophageal cancer found that improvement of dysphagia was equivalent with the two treatments.
A review of 119 patients treated with endoluminal palliation reported a significant improvement in dysphagia scores and an increased ability to relieve stenosis caused by tumor when PDT was used in conjunction with laser therapy and irradiation.
Other approaches include external-beam radiotherapy with or without an intracavitary brachytherapy boost, simple dilatation, placement of stents, and laser recannulization of the esophageal lumen.
Palliative resection for esophageal cancer is rarely warranted, although it does provide relief from dysphagia in some patients.
Chemotherapy Phase I and II studies have demonstrated moderate response rates to taxanes in esophageal cancer. Taxanes in combination with platinum compounds and fluoropyrimidines are being tested in regimens with irradiation.
Although chemotherapy alone may produce an occasional long-term remission, there is no standard regimen for patients with metastatic cancer. Patients with advanced disease should be encouraged to participate in well-designed trials exploring novel agents and chemotherapy combinations.
Chemotherapy in advanced esophageal cancer
In Britain, the ECF regimen, a combination of epirubicin (50 mg/m2) and cisplatin (60 mg/m2), both repeated every 21 days, with continuous infusion of 5-FU (200 mg/m2/d), is considered to be a standard regimen for advanced esophagogastric cancers. However, in the remainder of the world, there is no regimen that is considered to be the standard treatment of metastatic esophageal cancer. Difficulties in determining optimal therapy for this disease include the possible differences between esophageal squamous cell cancers and adenocarcinomas. Moreover, most of the available data regarding the treatment of metastatic esophageal cancer are derived from studies in which most patients had gastric cancer or from small phase II studies.
Although the regimen has been fairly well tolerated, infusional 5-FU has rendered the combination unpopular in other countries. Several phase III studies have been performed and consistently demonstrated objective responses in about 40% of patients, with a median survival of 9 months and a 1-year survival of 36% to 40%. The main severe toxicities of this regimen are neutropenia, in about one-third of patients (32%–36%), lethargy (18%), and nausea and vomiting (11%–17%). The REAL-2 study evaluated 1,002 patients (60% esophageal or GE junction cancer) with advanced esophagogastric cancers. They were randomly assigned to receive epirubicin (50 mg/m2 every 21 days) with either cisplatin (60 mg/m2) or oxaliplatin (Eloxatin; 130 mg/m2) every 21 days, and either infusional 5-FU (200 mg/m2/d) or capecitabine (Xeloda; 625 mg/m2 twice daily). In this study, the outcomes were similar in the resultant treatment groups, with median survivals around 10 months and 1-year survivals between 39% and 45%. Indeed, the single best-performing arm was EOX (epirubicin, oxaliplatin, Xeloda [capecitabine]), with a median survival of 11.2 months and a 1-year survival of 46.8%, although these results were not significantly superior to the other arms.
With the advent of many new chemotherapeutic agents (the taxoids, paclitaxel and docetaxel [Taxotere], irinotecan, and gemcitabine [Gemzar]) with varying mechanisms of activity, further studies have been conducted, and each of these drugs has demonstrated activity, with responses achieved in approximately 15% to 30% of patients.
However, the primary route of investigation for these new agents has been in combination with cisplatin and/or 5-FU. The results available to date suggest promising activity, with response rates often around 50% in phase II studies. Irinotecan (65 mg/m2) and cisplatin (30 mg/m2) administered weekly for 4 weeks every 6 weeks have also been active, with responses in 20 of 35 patients (57%) and an impressive median survival of 14.6 months. A Korean phase II study did not confirm these results but did suggest the combination was active, with a response rate of 31% of 32 patients, and a median survival of 9.6 months.
Following the example of the combination of irinotecan and 5-FU/leucovorin in colon cancer, the investigators have explored a modification of that schedule, with therapy administered for 2 weeks, with cycles repeated every 3 weeks. This simple modification has been investigated in 27 patients and was well tolerated, with severe neutropenia in 18% of patients and severe diarrhea in 11% of patients.
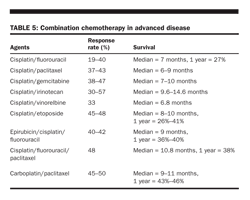
Paclitaxel (180 mg/m2 over 3 hours) and cisplatin (60 mg/m2 over 3 hours) administered every 14 days have been extensively evaluated in Europe. They were reported to produce objective responses in 43% of 51 patients, including two complete responses, and 43% of patients were alive 1 year after initiation of therapy (Table 5).
Because of the toxic and logistic difficulties of using cisplatin, carboplatin has become a popular chemotherapeutic drug. Several studies of carboplatin with paclitaxel in esophageal cancer have been undertaken. El-Rayes et al administered carboplatin (at an area under the concentration-time curve [AUC] of 5) with paclitaxel (200 mg/m2 over 3 hours) every 3 weeks in 33 chemotherapy-naive patients. Objective responses were reported in 45% of patients, with a median survival of 9 months and 1-year and 2-year survival rates of 43% and 17% respectively.
Polee et al explored a weekly schedule of these drugs in a phase I study. With therapy administered for 3 consecutive weeks, followed by a 1-week break, a dose of carboplatin (at AUC 4) with paclitaxel (100 mg/m2) was recommended for further investigation. Responses were noted in half of the 40 patients, with a median survival of 11 months and a 1-year survival of 46%. Both of these combinations were well tolerated, with the primary toxicity of myelosuppression.
Lorenzen et al treated 24 patients with esophageal and esophagogastric carcinomas and measurable disease with docetaxel (75 mg/m2 IV) and capecitabine (1,000 mg/m2 orally twice daily from days 1–14), with cycles repeated every 3 weeks. Only seven of the patients had adenocarcinomas, and eight had received prior chemotherapy. This combination had interesting antitumor activity, with objective responses in 11 patients (46%), including 56% of previously untreated patients and 2 of 8 patients who had received prior chemotherapy. Although patient numbers were small, there was no clear difference in response rates by histology. The therapy was reported to be fairly well tolerated overall, although dose reductions were necessary in 41% of patients. The main grade 3 and 4 toxicities were neutropenia (42% of patients, including two patients with neutropenic fever), diarrhea and neuropathy (13% of patients each), and hand-foot syndrome in 29% of patients.
The combination of docetaxel, cisplatin, and infusional 5-FU (DCF) has been approved by the U.S. FDA for the treatment of metastatic gastric and GE junction adenocarcinomas. The activity of this regimen in esophageal cancers is unclear. Chiarion-Sileni et al reported a study that provides some insight into the potential activity of this regimen. They studied a modification of the DCF regimen, with cisplatin (75 mg/m2) and docetaxel (60 mg/m2) given on day 1 and 5-FU (750 mg/m2/d) given on days 2 to 5 every 21 days for 3 cycles in patients with locally advanced esophageal cancer. This regimen was followed by radiation therapy with concurrent carboplatin. The response rate to chemotherapy alone with this regimen was 48% in 37 patients, suggesting that this combination is active in esophageal cancers as well.
The antimetabolite gemcitabine has also been evaluated in combination with cisplatin in esophageal cancer. A SWOG study, reported by Urba et al, combined 1,000 mg/m2 of gemcitabine on days 1, 8, and 15 with 100 mg/m2 of cisplatin on day 15 in 64 patients. Approximately one-quarter of these patients had received prior chemotherapy. Therapy was well tolerated, with severe neutropenia occurring in 31% of patients. The median survival of patients treated on this study was 7.3 months, and the 1-year survival rate was 20%. However, the heterogeneity of the patient population makes the efficacy of the therapy somewhat difficult to assess.
Oxaliplatin may also have a role in the treatment of esophageal cancer, both as a radiosensitizer and an agent in advanced disease. Mauer and colleagues reported the results of treatment with oxaliplatin, 5-FU, and leucovorin according to the FOLFOX4 schedule (oxaliplatin, 85 mg/m2 on day 1; leucovorin, 500 mg/m2 over 2 hours on days 1 and 2; and 5-FU, 400 mg/m2 bolus, then 600 mg/m2 over 22 hours on days 1 and 2, repeated every 14 days). Of 35 patients who were treated, objective responses were noted in 40%. The median survival rate was 7.1 months, the 1-year survival rate was 31%, and the 2-year survival rate was 11%. The median progression-free survival was 4.6 months. Although differences in patient populations were noted, these results are similar to those of other reported combinations.
The combination of oxaliplatin (130 mg/m2 on day 1) with capecitabine (1,000 mg/m2 twice daily on days 1 to 14) repeated every 21 days has also been studied in esophageal cancer. Thirty-eight percent of the 51 treated patients had objective responses, with a median survival of 8 months and a 1-year survival rate of 26%. This study was conducted in the Netherlands, and the tolerability and efficacy of this regimen in American patients remain to be determined.
The primary toxicity of these regimens is severe neutropenia, occurring in about 40% to 70% of patients. Severe diarrhea, nausea, and vomiting occur in ~10% to 15% of patients in many studies. Fatigue and asthenia also were significant side effects with both therapies.
With improving toxicity profiles and modest improvements in therapeutic outcomes, second-line therapy for advanced esophageal cancer is also increasingly being explored. Muro et al treated 28 Japanese patients with squamous cell carcinoma of the esophagus with docetaxel (70 mg/m2) every 3 weeks; these patients had previously received cisplatin and 5-FU. As expected, severe neutropenia was the dominant toxicity (88%, including nine episodes of febrile neutropenia), with severe anorexia, fatigue, and anemia also reported. Objective responses were noted in 16% of these patients.
In addition, Lordick et al determined that irinotecan (55 mg/m2) and docetaxel (25 mg/m2) given on days 1, 8, and 15, with cycles repeated every 28 days, were tolerable, with severe asthenia in 21% of 24 patients and severe diarrhea in 13%. However, only three partial responses (13%) and eight patients with stable disease were noted, with a resultant median survival of 26 weeks. Although these studies suggest the feasibility of second-line cytotoxic chemotherapy in esophageal cancer, the significant toxicity and limited objective response rate warrant its use only with caution and preferably on a clinical study.
Oral tyrosine kinase inhibitors of the epidermal growth factor receptor (EGFR) have also been investigated in esophageal cancer. Gefitinib (Iressa), at a daily dose of 500 mg, as second-line therapy in patients with esophageal adenocarcinomas was evaluated by Ferry et al. It produced partial responses in 11% of 27 patients, and an additional 7 patients had stable disease. In patients with esophageal cancer that had previously been treated with platinum-based chemotherapy, Janmaat et al treated 37 patients with the same regimen. They reported a confirmed response in only 1 patient, and stable disease in another 10, although 18% of patients were free of disease progression at 6 months, suggesting that some patients indeed experience disease control and benefit from this therapy. However, Radovich et al reported only 1 response in 23 patients treated with erlotinib (Tarceva; 150 mg daily). The toxicities were as expected, including rash, diarrhea, vomiting, and elevation in transaminase levels. As with lung and colon cancers, the optimal population for treatment with these targeted agents remains to be defined.
The other class of agents that target the EGFR pathway, monoclonal antibodies such as cetuximab, has provided mixed results in patients with esophageal cancer. For example, a German study of cisplatin/5-FU with or without cetuximab (Erbitux) as initial therapy in patients with metastatic esophageal squamous cell cancers with EGFR expression by immunohistochemistry suggested an improvement in patient outcomes. Sixty-two patients were randomized in a non-blinded fashion on the study, and crossover to cetuximab with cisplatin/5-FU was allowed in the patients who received initial cisplatin/5-FU alone. The response rates were similar (33% with cetuximab, vs 30%) in the two arms, but patients who received the additional cetuximab had a superior disease-control rate (75% vs 57%), median time to disease progression (5.9 months vs 3.6 months), and median overall survival (9.5 months vs 5.5 months). Moreover, of the five patients who crossed-over to second-line cetuximab, two had partial responses (one with single-agent therapy), and one had stable disease. However, given the sample size, and the unblinded nature of the study, with crossover, caution must be exercised when interpreting these results.
The SWOG studied cetuximab as a single agent as second-line therapy for metastatic esophageal adenocarcinoma. Of the 55 eligible and evaluable patients, only 1 had a partial response (2%), and 6 (11%) had stable disease. The median progression-free survival was 1.8 months, the median survival was 4.0 months, and the 6-month survival rate was 36%. The authors concluded that although the drug was well tolerated as single-agent therapy it did not meet its primary endpoint, and so could not be recommended as second-line therapy in esophageal cancer. The discordant results represented in these studies, between histologies and drugs, may reflect the differing biologies of esophageal adenocarcinomas and squamous cell carcinomas. Alternatively, they may reflect alterations in cancers after undergoing chemotherapy.
In addition to flavopiridol and the EGFR antagonists, it is anticipated that other targeted therapies, such as vascular endothelial growth factor antagonists, will be evaluated in this disease. Such avenues of exploration, in addition to early diagnosis and therapy for early-stage disease, are the most likely path toward significant improvements in therapy for esophageal cancer.
References:
SUGGESTED READING
Bedenne L, Michel P, Bouche O, et al: Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25:1160â1168, 2007.
Burmeister BH, Smithers BM, Gebski V, et al; Trans-Tasman Radiation Oncology Group; Australasian Gastro-Intestinal Trials Group: Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised, controlled phase II trial. Lancet Oncol 6:659â668, 2005.
Chiarion-Sileni V, Corti L, Ruol A, et al: Phase II trial of docetaxel, cisplatin and fluorouracil followed by carboplatin and radiation in locally advanced oesophageal cancer. Br J Cancer 96:432â438, 2007.
Cunningham D, Starling N, Rao S, et al: Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36â46, 2008.
Ferry DR, Anderson M, Beddard K, et al: A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: Evidence of gene expression, cellular, and clinical response. Clin Cancer Res 13:5869â5875, 2007.
Gold PJ, Goldman B, Iqbal S, et al: Cetuximab as second-line therapy in patients with metastatic esophageal cancer: A phase II Southwest Oncology Group Study. J Clin Oncol 26:abstract 4536, 2008.
Igaki H, Kato H, Ando N, et al: A randomized trial of postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus neoadjuvant chemotherapy for clinical stage II/III squamous cell carcinoma of the thoracic esophagus (JCOG 9907). J Clin Oncol 26:abstract 4510, 2008.
Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, et al: Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol 24:1612â1619, 2006.
Lee DH, Kim HT, Han JY, et al: A phase II trial of modified weekly irinotecan and cisplatin for chemotherapy-naive patients with metastatic or recurrent squamous cell carcinoma of the esophagus. Cancer Chemother Pharmacol 61:83â88, 2008.
Lordick F, Lorenzen S, Al-Batran S, et al: Cetuximab and cisplatin/5-FU (CF) versus CF in first-line metastatic squamous cell carcinoma of the esophagus (MESCC): A randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). J Clin Oncol 26:abstract 4546, 2008.
Lorenzen S, Duyster J, Lersch C, et al: Capecitabine plus docetaxel every 3 weeks in first- and second-line metastastic oesophageal cancer: Final results of a phase II trial. Br J Cancer 92:2129â2133, 2005.
Mauer AM, Kraut EH, Krauss SA, et al: Phase II trial of oxaliplatin, leucovorin, and fluorouracil in patients with advanced carcincoma of the esophagus. Ann Oncol 16:1320â1325, 2005.
Santharalingam M, Moughan J, Coia LR, et al: Outcome results of the 1996â1999 patterns of care survey of the national practice for patients receiving radiation therapy for carcinoma of the esophagus. J Clin Oncol 23:2325â2331, 2005.
Stahl M, Stuschke M, Lehmann N, et al: Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23:2310â2317, 2005.
Van Cutsem E, Moiseyenko VM, Tjulandin S, et al: Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 24:4991â4997, 2006.
van Meerten E, Eskens FA, van Gameren EC, et al: First-line treatment with oxaliplatin and capecitabine in patients with advanced or metastatic oesophageal cancer: A phase II study. Br J Cancer 96:1348â1352, 2007.
Abbreviations in this chapter
CALGB = Cancer and Leukemia Group B; JCOG = Japanese Cooperative Oncology Group; SWOG = Southwest Oncology Group