Capecitabine Has Unique Qualities That May Make It A Suitable Substitute for 5-FU
This special supplement to Oncology News International includes 28 reportswith updated information on clinical trials investigating capecitabine and other agents inthe treatment of advanced colorectal and breast cancers, and other solid tumors.The reports summarize selected presentations from the 39th Annual Meeting of theAmerican Society of Clinical Oncology (ASCO) and related educational symposiaheld in conjunction with ASCO.
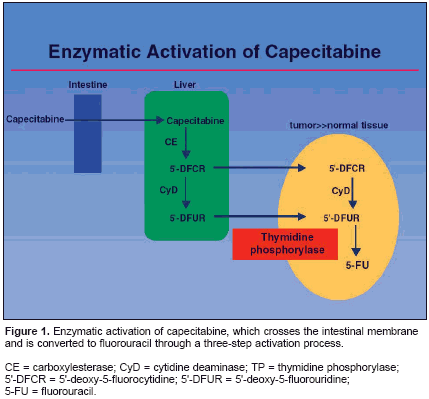
CHICAGO-The pharmacology ofcapecitabine (Xeloda) exhibits similaritiesto fluorouracil (5-FU) that make it asuitable substitute to 5-FU infusion, butcapecitabine also has unique qualities thatmake it a distinct drug. These attributesinclude several pharmacologic effects,such as a selective effect that results in ahigher concentration of 5-FU at the tumor,a potential for more effective interactionwith several chemotherapy agentsand radiation, and advantageous effectson genes involved in apoptosis and DNArepair. Furthermore, when capecitabineand radiation are combined, a relativelydramatic synergistic effect is achievedcompared with the use of either alone. Nosuch effect is observed with a combinationof radiation and 5-FU.These conclusions were reported byRobert Diasio, MD, of the University ofAlabama, Birmingham, at a symposiumsponsored by Roche on integratingcapecitabine in the management of colorectalcancer. Dr. Diasio listed severalproperties of capecitabine that distinguishit from 5-FU. "The compound has excellentbioavailability: it crosses the intestinalmembrane and then is convertedthrough a three-step activation process(see Figure 1). The third step involvesthymidine phosphorylase (TP), which ispresent more in tumor than in normaltissue, and accounts for much of the selectivityof this drug," Dr. Diasio said.The goal in developing capecitabinewas to create an agent that would workspecifically at the tumor site, and therebymaximize antitumor activity and improvetolerability. "The oral drug capecitabinehas many potential advantages. These benefitsinclude generating 5-FU in both plasmaand cells for a longer duration than IVbolus. Many of the complications associated with IV administration can be avoidedwhen an oral drug is used; and oraldrug administration is more convenient."When the mean ratio of 5-FU concentrationsfollowing capecitabine administrationare assessed, results indicate a significantlocalization of 5-FU within thetumor compared with concentrations inhealthy colon or rectal tissues or in plasma."This indicates that capecitabine reallygets to the point where you want it tobe maximally present-within the tumoritself," Dr. Diasio said."It is worth noting that the very importantprocesses of apoptosis and DNArepair are affected by capecitabine," Dr.Diasio added."One of capecitabine's most importantattributes is that the activation of thedrug can be upregulated by some of theother therapies that we use. A number ofdrugs have the ability to induce TP, andwhen they are used in combination withcapecitabine, we achieve a selective effectin terms of upregulation."
Rationale for XELOXDr. Diasio also outlined the rationalefor developing the XELOX regimen(capecitabine plus oxaliplatin [Eloxatin])and some aspects that make this a desirablecombination:
- These two drugs have differentmolecular mechanisms of action.
- A superior response rate and timeto progression are observed with the additionof oxaliplatin to 5-FU/leucovorin
- There are no overlaps in key toxicities.
- There is evidence of synergistic activitybeyond additive effects.
"Looking at xenograft studies, capecitabinealone or oxaliplatin alone has asimilar effect, but both taken together (attwo-thirds of the maximum tolerateddose) actually results in an effect that isbeyond an additive effect alone-it is asuper-additive effect," Dr. Diasio said.
Combined With Radiation
Radiation can also upregulate TP intumor tissue and not in adjacent normaltissue, and it works specifically at the lastselective enzymatic step in the activationprocess. "If we look at the effect of radiationon TP levels in the tumor, there is adose-related effect even from a single doseof radiation therapy,"Dr. Diasio said."This presents the possibility of minimizingradiation damage while increasing thechemotherapy effect. When we combinecapecitabine and radiation therapy, weget a relatively dramatic effect comparedwith either one used alone. After 35 days,this achieves a level of change that is beyondsuper-additive; it is synergistic. Thereis not such an effect seen with radiationtherapy and 5-FU."Dr. Diasio also notes that unlike 5-FU,capecitabine has a selective effect in termsof apoptosis. It inhibits Bcl2, a gene thatblocks tumor cell death induced by theradiation and chemotherapy agents. Also,capecitabine has a greater effect than5-FU in terms of inhibiting ERCC1, animportant DNA repair gene that alsoblocks the effect of radiation andoxaliplatin.
Investigating Properties
Trials designed to further investigatethe properties of capecitabine, particularlyin combination regimens in the treatmentof colorectal cancer were describedby Leonard Saltz, MD, of Memorial Sloan-Kettering Cancer Center. He pointed outthat phase I and II studies of capecitabinebasedcombinations in colorectal cancer show encouraging activity and a tolerablesafety profile. However, at present there isno randomized phase III data regardingthe use of capecitabine in combinationwith irinotecan (CPT-11, Camptosar),oxaliplatin (Eloxatin), or radiation therapy,and no phase III comparisons ofcapecitabine vs an infusional 5-FU regimenhave been reported.
CapOx vs FOLFOX
Dr. Saltz discussed two studies comparingcapecitabine/oxaliplatin (CapOx)to one of the FOLFOX regimens (fluorouracil/leucovorin/oxaliplatin) as firstlinetherapy for colorectal cancer. A studysupported by Roche is evaluating CapOxvs FOLFOX-4 among 1,000 patients. Theprimary objective is time to progression,and the secondary end points are overallsurvival, response rate, time to treatmentfailure, duration of response, tolerability,medical care use, and convenience. "Thisstudy will be looking at biomarkers andpharmacokinetics, and, to the degree thatit can be obtained, pharmacogeneticanalysis as well," Dr. Saltz said.A study from the Southwest OncologyGroup (SWOG) will assess CapOx vsmodified FOLFOX-6 (with 85 mg/m
2
oxaliplatin).The study will involve a largerpatient population (with an accrual goalof 1,730). The primary end point of theSWOG trial is overall survival.In addition, the two studies togetherwill indirectly provide a comparisonbetween FOLFOX-4 and the modifiedFOLFOX-6. The studies will be conductedin the United States, Europe, and elsewhere.
FOLFIRI vs IFL vs CapIri
A three-arm study is comparing FOLFIRI(fluorouracil, oxaliplatin, irinotecan)vs IFL on a 2-weeks on, 1-week off schedulevs CapIri (capecitabine, irinotecan).This study will look at whether giving IFLon a 2-week on, 1-week off basis will improvethe tolerability of the regimen, andprovide adequate efficacy. "The rationaleis that if there is lower toxicity, patientsmay stay on the drug longer and thenperhaps they will do better," Dr. Saltzsaid. "The 2-week on, 1-week off regimenwill probably be easier on the patient.Whether it will also be easier on the tumoris something this trial will address."
The X-ACT Study
Other studies are investigating whethercapecitabine should replace 5-FU/leucovorinin the adjuvant treatment of coloncancer. The X-ACT study is anopen-label, multicenter, phase III trialevaluating capecitabine vs the Mayo Clinicbolus 5-FU/leucovorin schedule. Disease-free survival is the primary end point,and a primary objective is to demonstrateat least equivalence. Secondary end pointsinclude overall survival, safety, quality oflife, health economics, and biochemicalmarkers.This study opened in 1998 and reachedits targeted recruitment goal with 1,987patients in 164 centers in 25 countries.Safety data were presented at this year'sAmerican Society of Clinical Oncologyannual meeting and efficacy data will bepresented at next year's meeting.
Capecitabine + Radiation forTreating Rectal Cancer
Another study, the National SurgicalAdjuvant Breast and Bowel Project(NSABP) R-04 schema, is addressing thequestion of whether capecitabine shouldreplace 5-FU/leucovorin in conjunctionwith radiation for rectal cancer. Patientswith T3-4 operable rectal cancers will bestratified for various risk factors and willreceive radiation therapy either withcapecitabine or infusional protracted5-FU."What is a little disquieting," Dr. Saltznoted, "is that the dose limiting toxicitywhen the capecitabine was combined withradiation was hand-foot syndrome. This'must give us pause.' The wrong thing todo is to adopt it routinely. The right thingto do is to conduct the trial."In summarizing, Dr. Saltz said, "Thereis extensive ongoing phase III testing ofcapecitabine-based combinations incolorectal cancer. Trials are going to bewidely available. There won't be manypeople without access to the trials. Andentrance to the trials should be stronglyencouraged."