XELOX Prior to Chemoradiation and Total Mesorectal Excision Produces Almost Universal Tumor Regression
This special supplement to Oncology News International includes 28 reportswith updated information on clinical trials investigating capecitabine and other agents inthe treatment of advanced colorectal and breast cancers, and other solid tumors.The reports summarize selected presentations from the 39th Annual Meeting of theAmerican Society of Clinical Oncology (ASCO) and related educational symposiaheld in conjunction with ASCO.
LONDON-Capecitabine (Xeloda)and oxaliplatin (Eloxatin), in combination(XELOX), prior to synchronouschemoradiation and total mesorectal excision(TME), produces almost universaltumor regression, rapid symptomatic response,and allows R0 resection in patientswith poor risk/locally advanced rectalcancer. These results were reported byIan Chau, MRCP, of the Royal MarsdenHospital, London and Surrey, UnitedKingdom (ASCO abstract 1087)."After neoadjuvant treatment withcapecitabine/oxaliplatin followed bychemoradiation, tumor response wasachieved in all patients, "Dr. Chau reported."All patients then had R0 resectionwith tumor regression away from the circumferentialresection margin (CRM),except for one inoperable patient."TME has been adopted as the standardtechnique in rectal cancer by surgeons inseveral European countries. CRM involvement,defined as tumor observed in lessthan or equal to 1 mm from the resectionmargin, is an important prognostic factorresulting in higher rates of local recurrenceand poorer survival in rectal cancersurgery.High-resolution, thin-slice magneticresonance imaging (MRI) has been shownto accurately stage rectal cancer and predictpotential CRM. MRI can provide anobjective method to define poor risk rectalcancer, identify patients most likely tobenefit from a preoperative treatmentstrategy, and assess primary tumor response.Poor Risk FactorsIn this study, patients were judged tohave poor risk rectal cancer if there were:tumors within 1 mm of mesorectal fascia,T3 tumors at/below levators, tumors extendinggreater than or equal to 5 mminto perirectal fat, T4 tumors, andT1-4N2 tumors. All patients on the studyhad tumor threatening CRM.
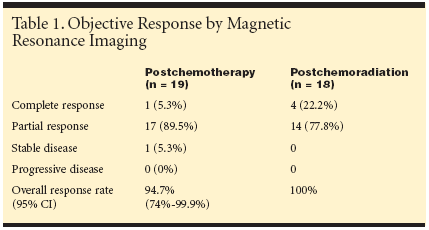
"In our previous study, neoadjuvantprotracted venous infusion fluorouracil(5-FU) and mitomycin (Mutamycin) as aprelude to synchronous chemoradiationwas administered with negligible risk ofprogression in the primary tumor andevolution of metastatic disease. This approachproduced considerable symptomaticresponse with associated tumor regression as neoadjuvant treatment," Dr.Chau said. "Oxaliplatin and capecitabinehas been shown to have promising activityas first-line treatment in metastaticcolorectal cancer and may provide betterefficacy than 5-FU and mitomycin as aneoadjuvant treatment."Treatment and ResponsePatients received 12 weeks of neoadjuvantcapecitabine 1,000 mg/m2 bid po for 14 days and oxaliplatin 130 mg/m2 IVevery 3 weeks. Starting on week 13, capecitabinewas continued at 825 mg/m2 bidcontinuously with concomitant radiotherapy45Gy in 25 fractions followed by a 5.4-9 Gy boost to the primary tumor. TMEwas planned 6 weeks after chemoradiation.Postoperatively, patients received 12weeks of capecitabine at 1,250 mg/m2 bid.MRI was repeated after chemotherapy andchemoradiation.From November 2001 to November2002, 22 patients were recruited. Patientshad a median age of 62 years (range 38-80), and a Eastern Cooperative OncologyGroup (ECOG) performance status of 0(27.3%) or 1 (72.7%).Following neoadjuvant capecitabine/oxaliplatin, the objective response ratewas 94.7%. In addition, 93% of patientshad symptomatic responses in a medianof 23 days including reduced rectal bleeding(100%), improvement in diarrhea/constipation (75%), diminished pelvicpain/tenesmus (93%), and weight gain/stabilization (100%).Following chemoradiation, tumor responsewas achieved in all patients (seeTable 1). Pathologic complete responsewas found in five patients (28%), and inan additional nine patients (47%), onlymicroscopic tumor foci were found insurgical specimens. Thirteen patients(68.4%) had downstaging of their primarytumor either in T (n = 3) or N (n = 3)or both (n = 7) staging."No patients developed progressivedisease after neoadjuvant chemotherapyor chemoradiation," Dr. Chau said. Onepatient died from myocardial infarctionand one from pulmonary embolism. Nograde 4 toxicity occurred during chemotherapyor chemoradiation.Significance of FindingsIn discussing the significance of thesefindings, Dr. Chau noted, "Despite themost optimal surgical approach in theform of total mesorectal excision and highqualitypreoperative radiotherapy, recentstudies have reported an unacceptablyhigh-15% to 20% rate of R1 resection-microscopic incomplete resection at themargin. Clearly, a more effective preoperativetreatment strategy needs to be implementedin order to improve both localcontrol and survival. Our study reports,for the first time, the implementation of apreoperative treatment strategy based onimaging assessment on the resection margin.Moreover, all patients with threatenedand involved circumferential resectionmargin based on preoperative MRIwere rendered margin negative on histologyafter treatment."