Cardiotoxicities of Breast Cancer Treatment
One of the potential side effects of chemotherapy is cardiac toxicity. The resulting damage to the heart can range from non–life-threatening events to devastating heart failure. The spectrum of these events can occur almost immediately, during a drug infusion, or as a delayed complication later in the patient’s life. Oncology nurses not only need to be familiar with identifying and intervening in acute cardiac events, but also in some instances will need to monitor for delayed cardiac toxicities during the continuum of the patient’s life.
One of the potential side effects of chemotherapy is cardiac toxicity. The resulting damage to the heart can range from non–life-threatening events to devastating heart failure. The spectrum of these events can occur almost immediately, during a drug infusion, or as a delayed complication later in the patient’s life. Oncology nurses not only need to be familiar with identifying and intervening in acute cardiac events, but also in some instances will need to monitor for delayed cardiac toxicities during the continuum of the patient’s life.
The mechanisms by which chemotherapy agents cause cardiac toxicities are as varied as the drugs themselves and are not clearly understood. The processes by which a patient can have an adverse cardiac event are multifaceted and depend on factors including the chemotherapeutic agent used, cumulative total dose of the agent, and the patient’s age and medical history of cardiac problems.[1] In general, cardiac events are categorized by drug class.
For example, it is theorized that anthracycline agents cause formation of free radicals, which leads to myocardial damage. The resulting cardiac damage can immediately cause transient arrhythmias, and delayed effects can result in heart failure (HF) or ventricular dysfunction.[2] In contrast, cardiotoxicites caused by antimetabolites are characterized by the drugs’ mediated cytotoxic action leading to an ischemic syndrome possibly induced by coronary vasospasm. Patients with this type of toxicity can experience chest pain, cardiac arrhythmia, or myocardial infarction, and the symptoms are usually reversible with discontinuation of the drug.[3]
Patients with breast cancer have the potential to receive several agents deemed to be cardiotoxic at various points during their therapy for this tumor type. In addition, some cardiotoxic therapies may be long-term, requiring clinicians and oncology nurses to be cautious and appropriately monitor and assess patients with breast cancer over long periods of time.[4]
Case Study
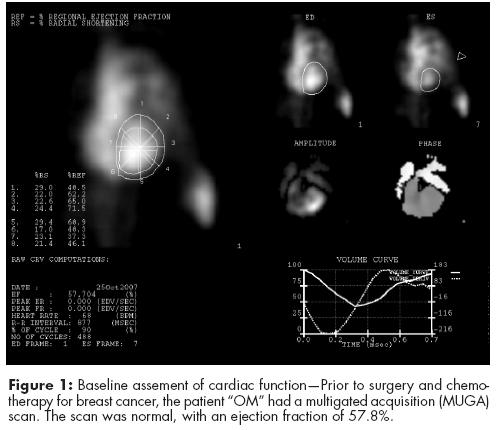
In August 2008, “OM,” a 49-year-old postmenopausal woman with a history of hypertension, presented with an enlarged left axillary lymph node. A mammogram was equivocal and a fine needle biopsy showed carcinoma. In September, she underwent surgical removal of the left axillary lymph nodes, assessment of which showed adenocarcinoma favoring breast as the primary site. Both a PET scan and CT scan showed no other metastatic sites, and prior to surgery and chemotherapy OM had a multigated acquisition (MUGA) scan. This scan was normal, with an ejection fraction of 57.8% (see Figure 1, p. 21).
Based on the findings, OM underwent a left breast mastectomy, and samples of the breast tissue showed a small focus of adenocarcinoma with the remaining lymph nodes tumor-free. Tumor staging was determined to be stage IIB (T1 N1 M0), estrogen receptor positive, progesterone receptor positive, and HER2 positive 3+.
The plan of treatment was four cycles of AC (anthracycline with cyclophosphamide) every 3 weeks, followed by docetaxel (Taxotere) and trastuzumab (Herceptin) for four cycles, followed by trastuzumab every 3 weeks for 1 year. The patient was able to complete therapy comprising AC, taxotere, and initial trastuzumab without significant problems or toxicities and she was scheduled to receive cycle six of her year-long therapy of trastuzumab.
At the next clinic visit, however, she complained of mild shortness of breath experienced while lying in bed at night, of approximately 1 month’s duration. Her physical examination was unremarkable except for mild 1+ pedal edema, and her chest X ray and a bone scan were normal. The patient underwent a repeat MUGA scan, which showed a decrease in the left ventricular contractility compared with the previous examination, from 57.7% to 47.7%. Trastuzumab was discontinued and a repeat MUGA scan was ordered in 3 months to determine further treatment options.
Cardiac Toxicity With Breast Cancer Treatment
Anthracyclines
Anthracycline toxicity is often termed type I cardiac dysfunction, which usually refers to the primarily left ventricular ejection fraction (LVEF) changes that can occur with use of this class of therapeutic agents. Because these changes can lead to heart failure and cardiomyopathy, and the risk increases with higher doses, oncology nurses must carefully monitor cumulative doses of anthracycline agents used in treatment of cancer.[1] Anthracycline-mediated cardiac damage usually is irreversible, in contrast to the cardiotoxicity associated with trastuzumab.[5]
Amelioration of anthracycline toxicity therefore is of great interest, and several strategies have been implemented to reduce this effect. Changing from bolus to continuous infusion has been studied and administration of cardioprotective agents, such as dexrazoxane, has been approved to reduce toxic effects of anthracyclines and other agents that can cause cardiac damage.[1]
Taxanes
Although cardiotoxicity has been noted with taxanes, particularly paclitaxel, the pathophysiology of this effect is not completely known.[1] Possible reasons for this toxicity include the glycerol-polyethylene glycol–containing solution used to improve the solubility of the agent, premedication used to prevent hypersensitivity reactions to the taxanes, or the effect of taxanes on cell microtubules.[6] The most frequently seen effects with taxanes include asymptomatic bradycardia, heart block, and cardiac ischemia, including changes in blood pressure and even reports of myocardial infarction.[2,7]
Trastuzumab
Trastuzumab is approved in the adjuvant and metastatic setting for patients with HER-2 positive, node-positive breast cancer. Cardiac toxicity is associated with use of this agent, however. Risk factors for trastuzumab cardiotoxicity are described in Table 1.[8] Toxicity is increased when trastuzumab is combined with anthracycline-based chemotherapy, therefore it is not recommended to give these two agents concurrently.
The cardiac damage seen with trastuzumab is termed type II, and it is linked to the endothelial growth factor receptor (EGFR) pathway responsible for cardiac contractility and function.[5]
A crucial difference between cardiotoxicity types I and II is that type II is largely reversible, and patients may show improvement in cardiac function after therapy is discontinued.[5] In one study of 38 patients with trastuzumab-induced toxicity, for example, the mean time to recovery of LVEF was 1.5 months after discontinuation of the monoclonal antibody. Re-treatment was possible in 25 patients, 88% of whom had no recurrence of LV dysfunction.[9]
Aromatase Inhibitors
Estrogen, despite its beneficial effect on lipid profiles in postmenopausal women, does not protect against ischemic heart disease.[10] Women who have breast cancer often receive anti-estrogen therapies in the treatment of their cancer, and tamoxifen was not shown to protect against ischemic heart disease in clinical trials; patients have higher rates of venous thromboembolism and stroke with this agent as well.[4] The aromatase inhibitor agents are very effective anti-estrogen therapies; one trial of letrozole, however, showed cardiac events were increased in the patient group receiving letrozole (Femara)[11]. This cardiotoxicity was not observed in trials of the other aromatase inhibitor drugs. Further research is needed to definitively answer questions regarding cardiotoxic effects and aromatase inhibitors.
Nursing Implications
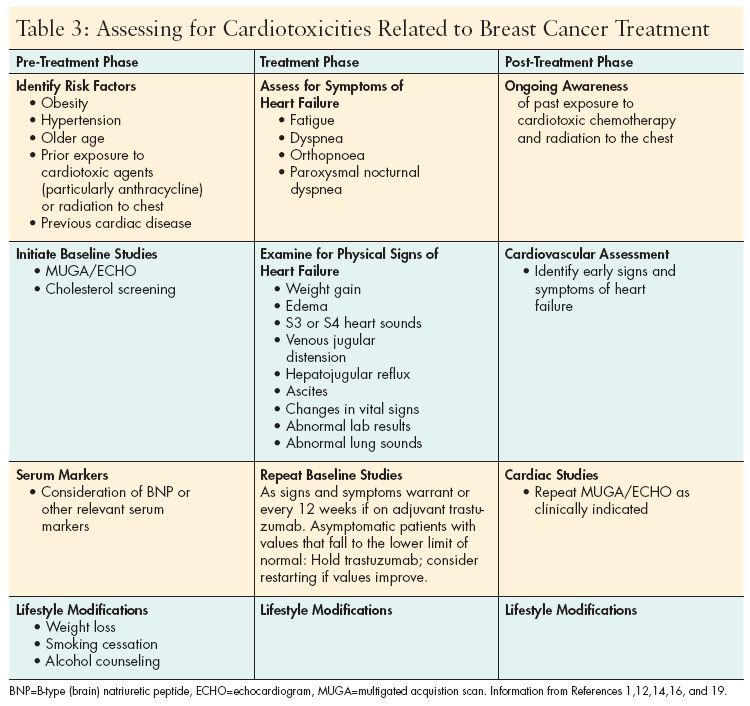
Oncology nurses need to be aware of the cardiotoxicities of antineoplastic drugs and understand that some events are transitory while others have irreversible consequences leading to significant morbidity or even mortality. They need to stratify patients’ risk factors for developing a cardiac event and be aware that advanced age, prior radiation therapy, prior chemotherapy, alcohol or cocaine abuse, and the existence of other comorbidities (ie, obesity, hypertension, hypercholesterolemia) are important to identify prior to initiating therapy.[1,12,13]
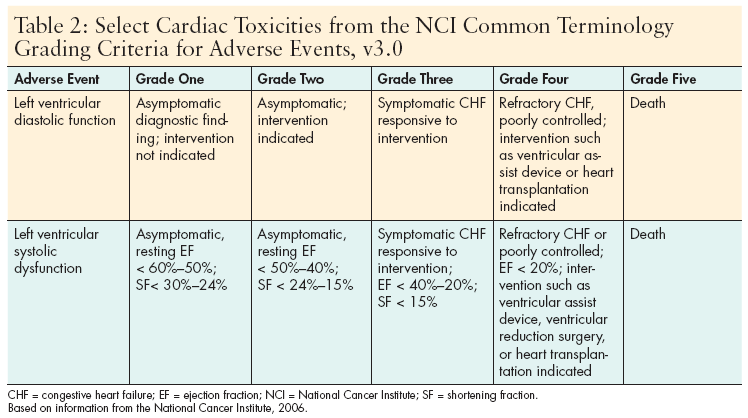
Assessment of hemodynamic and LVEF status prior to and during drug administration is essential to identifying potential risk for and development of heart failure in patients, and should continue into the post-treatment phase. (See Table 2, p.21 for National Cancer Institute grading criteria for selected adverse cardiac events.)
Patient assessment also should include checking for the presence of weight gain, edema, S3 or S4 heart sounds, venous jugular distention, hepatojugular reflux, ascites, crackles with auscultation of the lungs, and alterations in vital signs or laboratory results (electrolytes, blood urea nitrogen, creatinine).[1,2,14,15] The role of serum markers such as troponin and brain natriuretic peptide (BNP) as screening tools to identify heart failure related to trastuzumab is under investigation.[12,16]
Ultimately, the oncology nurse also needs to be skilled in distinguishing symptoms of early heart failure (fatigue, dyspnea, orthopnoea, paroxysmal nocturnal dyspnea) from symptoms attributed to cancer treatments.[15] Cardiac studies (echocardiogram [ECHO], electrocardiogram, and MUGA scans) will determine the extent of cardiac damage. If these studies show significant changes, patients will need to start additional therapies and make lifestyle changes.
The nurse’s role is to educate patients regarding these matters, to assess compliance, and to monitor patients for signs of impending or early failure or cardiac toxicity.[1] (See Table 3 for guidelines on cardiotoxcity assessment before, during, and after treatment for breast cancer.)
Case Study Resolution
The patient, OM, is scheduled to receive a repeat MUGA within 3 months of her last scan to determine if her cardiac function has improved. Although no ASCO (American Society of Clinical Oncology) clinical guideline exists, recommendations from the literature suggest that LVEF studies should be performed every 3 months, or sooner if the patient develops congestive heart failure (CHF).[16] Serial monitoring of the LVEF with MUGA or ECHO is considered to be the most practical way to effectively monitor for cardiotoxicity.[16]
Trastuzumab is held if the patient is asymptomatic and has a decline of LVEF to the lower limits of normal. It is restarted if LVEF returns to normal.[4] In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B31 clinical trial for adjuvant use of doxorubicin followed by paclitaxel with or without trastuzumab, treatment was discontinued in patients whose LVEF decreased by > 15% below the baseline value, in those whose LVEF remained below the normal value, and in patients with symptoms of CHF.[17]
As mentioned previously, reversibility of cardiac toxicity has been noted with trastuzumab and patients have been successfully rechallenged. If OM’s ejection fraction improves, it is possible that treatment with trastuzumab may be resumed in order to treat her with a full course of therapy. In making decisions about resumption of treatment, however, the risks of therapy with this agent must continue to be weighed against the absolute benefits.[18]
In addition, because this patient is ER and PR positive, she is a candidate for hormonal therapy, which carries a possible cardiotoxic risk. Further, if she develops recurrent or metastatic breast cancer and needs additional therapy, current NCCN guidelines note that while further trastuzumab combined with other first-line agents could be a treatment possibility, consideration of a less cardiotoxic regimen using lapatinib (Tykerb) and capecitabine (Xeloda) would be an option.[18]
Bevacizumab (Avastin), a vascular endothelial growth factor inhibitor agent approved for metastatic breast cancer in 2008, carries its own cardiovascular toxicity profile, including hypertension, myocardial infarction, changes in LVEF, and thrombosis, stroke, and heart failure.[12] In addition to the risk factors for cardiotoxicity already described in this article, use of polychemotherapy as well as radiation therapy in patients with breast cancer can increase cardiovascular toxic effects. Patients with this disease have ample opportunity for exposure to potential cardiotoxic therapies.
Conclusion
Cardiotoxicity can occur with several of the therapies integral to treatment of the patient with breast cancer. A multidisciplinary approach is suggested to manage patients with high cardiovascular risk.[19] Recommendations for monitoring of patients with breast cancer after therapy for this tumor type include following the LVEF with MUGA or ECHO before, during, and after trastuzumab treatment.[4]
Oncology nurses and other healthcare professionals must be vigilant in their initial and ongoing assessment of the patient with breast cancer for signs of cardiac failure or other cardiovascular toxic effects.
References:
1. Viale PH, Yamamoto DS:.Cardiovascular toxicity associated with cancer treatment. Clin J Oncol Nurs 12(4):627â638, 2008.
2. Yeh ET, Tong AT, Lenihan DJ, et al: Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation 109(25):3122â3131, 2004.
3. Becker, K, Erckenbrecht JF, Haussinger D, et al: Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 57(4):475â484, 1999.
4. Bird BR, Swain SM: Cardiac toxicity in breast cancer survivors: Review of potential cardiac problems. Clin Cancer Res 14(1):14â24, 2008.
5. Safra T: Chemotherapeutics and cardiac toxicity: Treatment considerations and management strategies. Community Oncol 4:540â548, 2007.
6. Jones RL, Ewer MS: Cardiac and cardiovascular toxicity of nonanthracycline anticancer drugs. Expert Rev Anticancer Ther 6(9):1249â1269, 2006.
7. Chanan-Khan A, Srinivasan S, Czuczman MS: Prevention and management of cardiotoxicity from antineoplastic therapy. J Support Oncol 2(3):252â256, 2004.
8. Pinder MC, Duan Z, Goodwin JS, et al: Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 25(25): 3808â3815, 2007.
9. Ewer MS, Vooletich MT, Durand JB, et al: Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol 23(31):7820â7826, 2005.
10. Rossouw JE, Anderson GL, Prentice RL, et al: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321â333, 2002.
11. Coates AS, Keshaviah A, Thurlimann B, et al: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol 25(5):486â492, 2007.
12. Jones LW, Haykowsky MJ, Swartz JJ, et al: Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 50(15):1435â1441, 2007.
13. Van Camp G: Toxic cardiopathies. Acta Clinica Belgica 60(5):293â300, 2005.
14. Bashore TM, Granger CB, Hranitzky P, et al: Heart, in McPhee ST, Papakakis MA, Tierney LM (eds): Current Medical Diagnosis and Treatment, pp 287â375. New York, NY, McGraw-Hill, 2009.
15. Fadol A: Management of acute decompensated heart failure in patients with cancer. Clin J Oncol Nurs 10(6):731â736, 2006.
16. Sengupta PP, Northfelt DW, Gentile F, et al: Trastuzumab-induced cardiotoxicity: Heart failure at the crossroads. Mayo Clin Proc 83(2):197â203, 2008.
17. Martin M, Esteva FJ, Alba E, et al: Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: Review and expert recommendations. Oncologist 14(1):1â11, 2009.
18. National Comprehensive Cancer Network (NCCN) (2009). NCCN Practice Guidelines in Oncology â v.1.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Accessed on February 6, 2009.
19. Lenihan DJ, Esteva FJ: Multidisciplinary strategy for managing cardiovascular risks when treating patients with early breast cancer. Oncologist 13(12):1224â1234, 2008.
20. Hurst JW, Morris DC, Alexander RW: The use of the New York Heart Association’s classification of cardiovascular disease as part of the patient’s complete Problem List. Clin Cardiol 22(6):385â390, 1999.