Cetuximab Is a Good Option When Standard Treatment Fails, Large Trial Reports
The 30 reports in this special supplement to Oncology News International represent highlights of ongoing major clinical trials and new research presented at ASCO 2004 regarding state-of-the-art chemotherapeutic management of gastrointestinal and other cancers. Important developments in capecitabine as adjuvant therapy, novel targeted agents, and new combinations are discussed.
LOS ANGELES-In patients whohave exhausted all other treatmentoptions for metastatic colorectal cancer,cetuximab (Erbitux) is well toleratedand active. This is the conclusiondrawn from one of the largest trials todate of single-agent monoclonal antibodytherapy in chemotherapy-refractorymetastatic colorectal cancer (abstract3510).A response rate of about 12% wasreported for cetuximab initiated innearly 350 patients in whom regimenswith irinotecan (CPT-11, Camptosar)and with oxaliplatin (Eloxatin) hadfailed. Median survival time approached7 months (abstract 3510).The study report, "demonstratesconvincingly that this agent has activityin heavily pretreated patients and iswell tolerated," reported Heinz-JosefLenz, MD, director of gastrointestinaloncology at USC/Norris ComprehensiveCancer Center, Los Angeles."A response rate of over 10% isimpressive: compare to that the responserates with FOLFOX [fluorouracil(5-FU)/folinic acid/oxaliplatin]and FOLFIRI [5-FU/folinic acid/irinotecan]in second-line chemotherapy,"Dr. Lenz stated.Interestingly, response was con-firmed in one of nine patients whosetumors had stained negative for epidermalgrowth factor receptor (EGFR).This was notable because the activityof cetuximab is thought to be relatedto its activity against EGFR. As a resultof this finding, which included oneconfirmed and one unconfirmed response,investigators launched a newtrial to evaluate the efficacy of cetuximabin EGFR-negative tumors.Impressive Response Rate
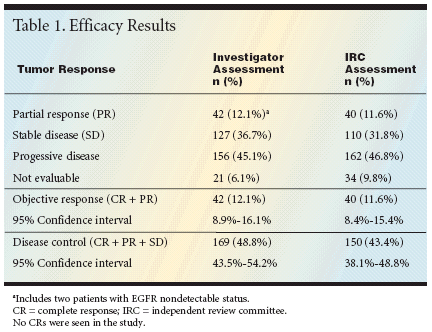
For the study, a total of 346 patientswith metastatic colorectal cancerreceived cetuximab at an initialdose of 400 mg/m2, followed by weeklytreatments at a dose of 250 mg/m2.The patients had undergone a medianof four prior regimens containingirinotecan or oxaliplatin. All but ninepatients had tumors with immunohistochemicalevidence of EGFR expression.The objective response rate was12.1% (42 patients, all partial responses)by investigator assessment and11.6% (40 patients) by independentreview committee (IRC) assessment(see Table 1). Another 31.8% (110patients) had stable disease by IRCassessment. The median overall survivaltime was 6.7 months.These results were consistent withprior studies of cetuximab monotherapyin metastatic colorectal cancer,according to the investigators.Safe, Well Tolerated
The most adverse event was anacneiform rash characteristic of treatmentwith an EGFR-targeted agent.The rash was seen in 90% of patientstreated, but in only 12% of cases wasthe rash grade 3/4 in severity, including2% grade 4. Infusion reactions,almost all grade 1/2, were noted in17% of patients. Other reported adverseevents included diarrhea, fatigueor malaise, nausea and vomiting, andmucositis/stomatitis.
"This is an effective agent in refractorypatients, and it is safe and welltolerated," Dr. Lenz said.Further investigation is warrantedin "EGFR nondetectable" tumors, investigatorsadded. Of the nine patientswith tumors in which EGFR could notbe detected, four had disease control,including the one confirmed responseand three patients rated as having stabledisease. In addition, eight of thenine patients had the characteristicacneiform rash.