Chemotherapy Benefit Confirmed in Advanced NSCLC
Madison, Wis-The largest randomized clinical trial ever conducted in advanced non–small-cell lung cancer (NSCLC) has shown that combination chemotherapy regimens extend survival in advanced disease.
Madison, WisThe largest randomized clinical trial ever conducted in advanced nonsmall-cell lung cancer (NSCLC) has shown that combination chemotherapy regimens extend survival in advanced disease.
The four different treatment regimens were equivalent in terms of most of the outcomes studied, with no one combination emerging as clearly superior to the reference regimen of paclitaxel (Taxol)/cisplatin (Platinol).
Results of the Eastern Cooperative Oncology Group (ECOG) trial 1594 were presented at the ASCO meeting by principal investigator Joan H. Schiller, MD, professor of medicine, University of Wisconsin, Madison.
The study involved 1,163 patients and compared three different treatment combinations with the standard paclitaxel/cisplatin regimen.
No Significant Differences
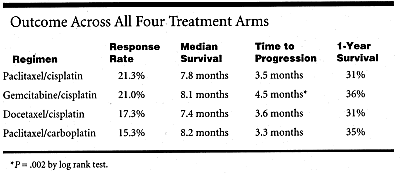
The analysis found no statistically significant differences between four commonly used chemotherapy regimens, in terms of overall response rate (18.7%), overall 1-year survival (33.5%), and overall 2-year survival (12%). The gemcitabine (Gemzar)/cisplatin arm, however, offered a statistically significant 1-month advantage in time to progression (4.5 months vs 3.5 months; P = .02) but was more toxic than the other arms, Dr. Schiller reported.
The activity of all four regimens indicates that a number of effective chemotherapy options are available to oncologists, and their selection should be based on factors other than their activity in NSCLC, Dr. Schiller said. The general message of ECOG 1594, she said, was that chemotherapys benefit was confirmed.
Improved Survival Rate
Survival for advanced lung cancer patients has improved with the use of chemotherapy as a treatment, Dr. Schiller said. Patients now have a 35% to 40% chance of 1-year survival, up from a 20% to 25% 1-year survival 5 years ago.
ECOG 1594 compared three platinum-based regimens containing third-generation drugs active against NSCLC to a reference regimen of 24-hour paclitaxel/cisplatin in stage IIIB and IV patients. The experimental arms were gemcitabine/cisplatin, docetaxel (Taxotere)/cisplatin, and carboplatin (Paraplatin)/paclitaxel.
Patients received cisplatin 75 mg/m² on day 1 plus paclitaxel 175 mg/m²/24 hours; gemcitabine 1,000 mg/m² on days 1, 8, and 15 plus cisplatin 100 mg/m² on day 1; docetaxel 75 mg/m² on day 1 plus cisplatin 75 mg/m² on day 1; or paclitaxel 225 mg/m²/3 hours on day 1 plus carboplatin AUC 6 on day 1.
The trial was based on results of an earlier ECOG trial (Bonomi et al: J Clin Oncol 18:623-631, 2000) that compared two dose levels of paclitaxel and cisplatin to cisplatin and etoposide. The lower-dose paclitaxel/cisplatin arm produced a higher response rate, better survival, and fewer side effects than the other two arms, and thus became the reference for this trial.
Reducing Side Effects
But todays standard in combination chemotherapy for NSCLC is actually paclitaxel/carboplatin, according to Joseph Treat, MD, medical director, Fox ChaseTemple University Cancer Center. He said this regimen was compared to paclitaxel/cisplatin to put to rest the suspicion that carboplatin may not be equivalent to cisplatin.
The studys primary objective was to determine which of four different types of chemotherapy treatment is best for patients with advanced lung cancer, Dr. Schiller noted. While all of the chemotherapy regimens used today are effective, only the paclitaxel plus carboplatin regimen has been shown to have statistically fewer life-threatening side effects.
Indeed, in ECOG 1594, the regimen of paclitaxel/carboplatin had the lowest rate of grades 3, 4, and 5 toxicities, including less nausea and vomiting, less need for IV antibiotics, and fewer hospitalizations.
If I had more advanced disease and was looking for a slightly more gentle regimen, I would go with paclitaxel/carboplatin, commented the discussant of the session, Frances Shepherd, MD, professor of medicine, University of Toronto.
The docetaxel/cisplatin arm was associated with the most hypersensitivity reactions. The gemcitabine/cisplatin arm was the most toxic in general, including renal toxicity, but this regimen included higher doses of cisplatin and was dosed more frequently than the other arms. Other than the variations in toxicity, the results were quite uniform across all treatment groups (see Table).
Time to Progression
The only statistically significant difference was the improved time to progression with gemcitabine/cisplatin. Within the whole study population, there was also a statistically significant difference in survival by performance status (PS), with PS 0 patients achieving a median survival of 10.7 months, compared to 4 months for PS 2 patients.
In discussing the study, Dr. Shepherd pointed out that many of the outcomes (see Table, above) were lower than results seen in similar trials of these combination regimens. The difference might be attributed to the inclusion of patients with poor PS and to the smaller proportion of stage IIIB patients in this study, compared with several European trials achieving higher response rates.
She also suggested that the lack of benefit from one arm vs the others in this study may have occurred because many patients crossed over to second-line therapy, and therefore essentially received the same treatment. This, unfortunately, will remain an unanswered question, she said.
Dr. Shepherd concluded, This race has a photo finish . . . Based on the results of this trial, do we have a new gold standard for the treatment of NSCLC? No, but I think we have several optionspaclitaxel/carboplatin, gemcita-bine/cisplatin, and, based on last years SWOG trial, vinorelbine (Navelbine)/cisplatin.
A future Southwest Oncology Group trial will evaluate the standard paclitaxel/carboplatin regimen vs a novel triplet of paclitaxel/carboplatin/tirapazamine (Tirazone), which was developed by Dr. Treat.
Tirapazamine has little activity as a single agent but works synergistically with cisplatin, he explained. Also, a multicenter coalition phase III trial chaired by Dr. Treat is evaluating paclitaxel/gemcitabine, paclitaxel/carboplatin, and gemcitabine/carboplatin in patients with metastatic stage IIIB or IV NSCLC.