Chemotherapy Dose Intensity and Quality Cancer Care
Myelosuppression and particularly neutropenia and associated complications, including febrile neutropenia, continue to represent the major dose-limiting toxicity of cancer chemotherapy.
Myelosuppression continues to represent the major dose-limiting toxicity of cancer chemotherapy. Neutropenia and its complications, including febrile neutropenia, occur most often early in the course of treatment but are often underreported in clinical trials. In addition to associated morbidity, mortality, and cost, myelosuppression is often associated with reduced chemotherapy dose intensity. Reduction in chemotherapy dose intensity may compromise disease control and survival in patients with curable malignancies. Several investigators have argued, based on retrospective reviews of large clinical trials, that myelosuppression and neutropenia are surrogates for delivered dose intensity with patients encountering neutropenic events uniformly experiencing better survival. While the number of randomized trials in which patients were deliberately assigned to reduced dose intensity are limited, they consistently demonstrate poorer long-term outcomes in patients with responsive and potentially curable malignancies. The design and implementation of such studies are limited by both ethical concerns and study power and sample size considerations. Recent interest in dose dense schedules also suggests that such an approach may further increase the proportion of patients surviving for 5 years or more. Despite the compelling data available, national practice pattern surveys in the United States have consistently demonstrated a high frequency of dose reductions and delays such that more than half of patients being treated with curative intent receive significantly reduced dose intensity. This review will summarize and discuss the reasons for such undertreatment and strategies available to avoid dose reductions and delays. Randomized controlled trials as well as meta-analyses of those trials have demonstrated that the myeloid growth factors, in addition to reducing the risk and consequences of febrile neutropenia, are capable of sustaining or increasing chemotherapy dose intensity. Recent economic studies suggest that due to the impact of greater relative dose intensity on long-term outcomes, growth factor support to sustain dose intensity is both an effective and cost-effective management strategy. The delivery of optimal chemotherapy dose intensity in patients with potentially curable malignancies should be considered a major quality indicator in cancer patient care.
Myelosuppression and particularly neutropenia and associated complications, including febrile neutropenia, continue to represent the major dose-limiting toxicity of cancer chemotherapy.[1] Although neutropenic complications are associated with substantial morbidity, mortality, and cost, they are also frequently associated with chemotherapy dose reductions and delays.[2-5] The reduction in treatment dose intensity can potentially compromise disease control and long-term survival in responsive and potentially curable malignancies. The myeloid growth factors or colony-stimulating factors (CSFs), have been shown to reduce the incidence, duration, and severity of neutropenic events and represent an alternative to chemotherapy dose attenuation.[6-9] Other causes of dose reductions and treatment delays include anemia and associated fatigue, thrombocytopenia and the associated risk of bleeding, as well as numerous nonhematologic toxicities such as nausea and vomiting, mucositis, and renal dysfunction.
Risk and Consequences of Chemotherapy-Induced Severe and Febrile Neutropenia
Risk of Neutropenic Events
The risk of febrile neutropenia in chemotherapy patients correlates directly with both the severity and duration of neutropenia, which in turn depend upon the specific regimen utilized and the dose intensity administered[10-11] The risk of hematologic complications in patients receiving chemotherapy has been systematically underreported in randomized controlled trials.[12] Recent data from a health-care claims database confirm rates of chemotherapy-related serious adverse events, most commonly related to neutropenic complications, that are greater than those reported by large clinical trials leading to more suffering and cost than previously thought.[13]
Timing of Neutropenic Events
The risk of the initial event for many regimens appears to be greatest during the first cycles of chemotherapy (Figure 1).[14-15] This is particularly true in elderly patients and those with important comorbidities where first-cycle risk may be severalfold greater than in patients without such comorbidities. In a nationwide prospective registry of adult cancer patients treated with chemotherapy in community oncology practices, approximately two-thirds of episodes of chemotherapy-induced neutropenia and febrile neutropenia were experienced in the first cycle of chemotherapy across a range of disease categories and chemotherapy regimens.[16] The reason for the lower risk after the first cycle appears to relate to dose reductions and delays or the initiation of a myeloid growth factor in subsequent cycles. When dose intensity is maintained and prophylactic agents are not added, the rates of severe or febrile neutropenia persist across multiple cycles with upwards of one-third of patients experiencing two or more events.[17] In studies performed before the availability of the CSFs, the risk of febrile neutropenia across multiple cycles of chemotherapy remained high when dose intensity was maintained.[18]
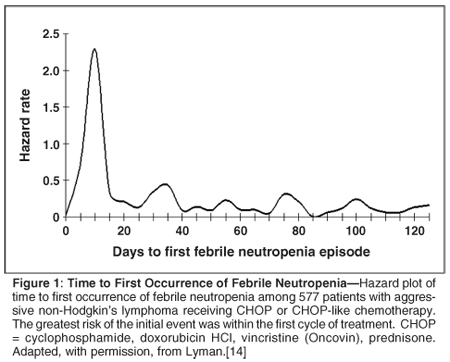
Consequences of Neutropenic Events
Most patients with febrile neutropenia require hospitalization for clinical evaluation and the administration of empiric, broad-spectrum antibiotics. It is estimated that approximately 60,000 patients with cancer and febrile neutropenia are hospitalized each year in the United States; inpatient mortality rates average from 7% to 11%.[2,3] Risk factors for inpatient mortality include gram-negative and gram-positive sepsis, pneumonia, fungal infection, leukemia, pulmonary embolism, hypotension or hypo-volemia on admission, and various comorbidities, including cardiac, cerebrovascular, renal, or liver disease.[2] In addition, costs associated with hospitalization for febrile neutropenia are high, with recent estimates ranging from $10,000 to $20,000 per hospitalization.[2] There are, in addition, nonmedical as well as indirect and out of pocket expenses that are very real costs to the patient and family.[19,20] Potentially the most serious complication of myelosuppression and associated neutropenia, however, is the impact on delivered chemotherapy dose intensity.[17]
Importance of Chemotherapy Dose Intensity
Definition of Relative Dose Intensity
By convention, chemotherapy dose intensity is defined as the dose given per unit weight or body surface area per unit time, eg, mg/m2/wk.[21,22] Often the chemotherapy dose intensity is expressed as relative dose intensity or the ratio of the dose intensity delivered to the standard dose intensity for a chemotherapy regimen. Reductions in relative dose intensity may be either planned from the beginning or unplanned usually due to toxicity that the patient experiences such as neutropenia and infection.[4,5]
Preclinical Studies of Dose Intensity
Evidence for the importance of dose intensity in patients receiving cancer chemotherapy comes from several sources. In cell culture, tumor growth correlates inversely with the chemotherapy drug concentration in the culture, or alternatively, the number of tumor cells killed correlates directly with the concentration of most chemotherapy drugs in the culture media.[23] Likewise, in animal tumor models, countless demonstration of a dose-response relationship with virtually all chemotherapy drugs studied has been shown. In these studies, a reduction in dose almost always results in a reduction in cure rates even before a decrease in response rate is observed. In fact, Skipper demonstrated that a 50% reduction in cure rates resulted from a 20% reduction in dose before a significant reduction in response was observed.[24] In humans, the data span the entire extent of disease observed.
Clinical Studies of Dose Intensity in Advanced Disease
A significant relationship has been observed between objective tumor response dose intensity in the metastatic setting. In patients with advanced disease, chemotherapy dose intensity correlates with tumor response rates, ie, the greater the dose intensity the higher the complete and partial response rate.[25,26]
Retrospective Clinical Studies of Dose Intensity in Curable Malignancies
In patients with early-stage disease, retrospective analyses of randomized controlled clinical trials have suggested strong association between the dose intensity actually given and disease-free as well as overall survival. A 20-year follow-up of the early Milan trial of adjuvant chemotherapy with cyclophosphamide, methotrexate, and fluorouracil (5-FU) in lymph node-negative early-stage breast cancer demonstrated that patients who received > 85% of planned dose intensity experienced better disease-free and overall survival than patients who received lower dose intensity (Figure 2).[27] Patients receiving less than 65% of standard dose intensity experiencing survival similar to that of untreated controls suggesting that the entire benefit of adjuvant chemotherapy may be lost by such levels of reduced dose intensity.
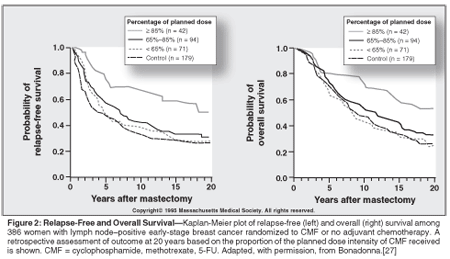
A recent retrospective study of four separate randomized controlled trials suggests that most of the survival impact of reduced dose intensity is evident in hormone receptor-negative disease.[28] Another retrospective analysis of the LNH-87 study reported that patients who received more than 70% of the planned dose intensity experienced significantly longer survival.[29] Epelbaum found that the 5-year overall survival was significantly greater in lymphoma patients who received cyclophosphamide, doxorubicin HCl, vincristine (Oncovin), and prednisone (CHOP) with a relative dose intensity > 70% compared to patients receiving < 70%.[30]
While several studies have demonstrated that older patients experience a greater risk of progressive disease following lymphoma chemotherapy, Lee et al demonstrated that no significant difference in survival was found among older lymphoma patients who received close to full dose intensity.[31] Similar data exist for several additional cancer types commonly treated with curative intent. Kwak reported that patients with non-Hodgkin's lymphoma (NHL) receiving 75% or less of standard chemotherapy dose intensity experienced significantly shorter survival than those receiving more than 75% relative dose intensity.[32] Related studies have demonstrated a significant adverse impact of delays in initiation of adjuvant systemic chemotherapy in elderly women with breast cancer.[33]
Myelosuppression as a Surrogate for Chemotherapy Dose Intensity
A number of studies have demonstrated that myelosuppression in general or leukopenia and neutropenia in particular encountered during a course of chemotherapy is significantly associated with improved survival across a range of human malignancies. Mayers reviewed the 15-year follow-up of 484 women who had received adjuvant chemotherapy for early-stage breast cancer stratified on the basis of whether they had experienced myelosuppression during their course of chemotherapy (Figure 3).[34] Myelosuppression remained a significant prognostic factor for improved survival after adjusting for stage, receptor status, and treatment (hazard ratio = 0.77; 95% confidence interval [CI] = 0.59-1.00). The evident myelosuppression is interpreted by most as a surrogate for delivered chemotherapy dose intensity, suggesting again a strong relationship between such dose intensity and clinical outcomes.
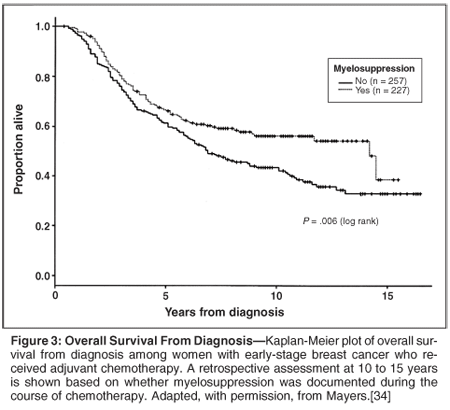
In a pooled analysis of 1,265 patients with non-small-cell lung cancer from three randomized trials, Di Maio reported that those with neutropenia during their chemotherapy experienced significantly longer survival.[35] Like other authors, they conclude that neutropenia is a surrogate marker for optimum dosing of chemotherapy. While other studies have observed very similar findings in a wide variety of settings, all of these studies are limited by their retrospective design.
Overview of Prospective Randomized Controlled Trials of Early-Stage Breast Cancer
The updated overview of all randomized controlled trials of adjuvant chemotherapy vs controls in early-stage breast cancer has demonstrated a significant reduction in 15-year recurrence rates, breast cancer mortality, and all-cause mortality.[36] Modern combination chemotherapy regimens reduced annual breast cancer death rates compared to untreated controls by 38% in women < 50 and 20% in women &ge 50 years of age. A dose-response relationship, therefore, must exist between a chemotherapy dose intensity of zero as in the controls and the dose intensity that was actually administered in the randomized controlled trials. Therapeutic benefit appears to be lost and survival compromised somewhere between the dose intensity actually received in the treatment arm compared to that of the control arm. The investigators suggest that even greater improvements in long-term survival may be achievable with better compliance as well as with the newer regimens now in use.[36]
Prospective Randomized Controlled Trials of Different Dose Intensities
Two randomized controlled trials of adjuvant chemotherapy in early-stage breast cancer where patients were deliberately randomized to different dose intensities have been reported. A study in patients with early-stage breast cancer (Cancer and Leukemia Group B [CALGB] 8541) randomized patients to three different relative dose intensities (1.0, 0.67, 0.50) of adjuvant cyclophosphamide, doxorubicin (Adriamycin), and 5-FU (Figure 4).[37] At a median follow-up of 9 years, patients in the low dose intensity arm experienced significantly worse disease-free (P < .0001) and overall survival (P = .004) than patients in the moderate- and full-dose intensity arms respectively. Similarly, a randomized trial conducted by the French Adjuvant Breast Cancer Group demonstrated a regimen of 5-FU, epirubicin (Ellence), and cyclophosphamide was superior in terms of 10-year disease-free (50.7% vs 45%; P = .036) and overall survival (54.8% vs 50.0%; P = .038) when epirubicin was given at a dose of 100 mg/m2 rather than 50 mg/m2, respectively.[38]
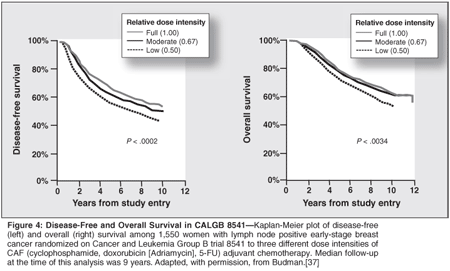
In addition, Italian investigators have reported a randomized phase II study in elderly patients with small-cell lung cancer showing 1-year survivals of 39% vs 18% in patients who received a full-dose regimen of platinum and etoposide with prophylactic CSF vs attenuated doses without CSF support.[39] Increasing dose intensity often is accompanied by bone marrow or peripheral blood stem cell support where patients are treated with very intensive regimens followed by an infusion of myeloid stem cells to repopulate bone marrow elements.
Clinical Studies of Increased Dose Intensity
Generally studies that increase dose intensity by increasing drug doses increase toxicity but have limited impact on cancer outcomes beyond a certain dose. However, dose-dense regimens with shortened treatment intervals requiring CSF support permits upwards of a 50% increase in relative dose intensity and have demonstrated improved survival over standard regimens in early-stage breast cancer and NHL.[40,41] At a median follow-up of 36 months, results from CALGB 9741 in node-positive early-stage breast cancer patients have demonstrated that dose-dense AC→T (doxorubicin [Adriamycin]/cyclophosphamide followed by paclitaxel [Taxol]) results in reductions in relative risk for disease-free survival and mortality of 26% (P = .0072) and 31% (P = .014), respectively.[40]
Likewise, a study of dose-dense chemotherapy in patients with NHL by the German High Grade Lymphoma Group has demonstrated significant improvements for CHOP-14 in time to progression and event-free survival as well as overall survival among older patients.[41] Such results were anticipated based on mathematical models developed by Larry Norton and Richard Simon predicting greater tumor cell kill with the compression of the chemotherapy schedule.[42] The theory is that when tumor cells are killed with chemotherapy, the remaining cells, which are smaller in number, start to grow at a faster rate. In principle, most chemotherapy drugs are more effective against rapidly growing cells. It was suggested that by treating more frequently, a greater proportion of rapidly growing cells may be killed, preventing rapid regrowth of tumors between cycles of therapy.
Why Have There Not Been More Controlled Clinical Trials?
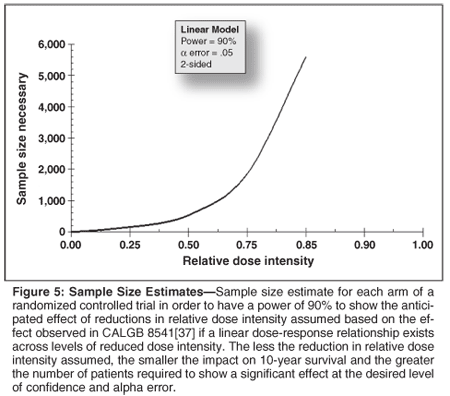
It is reasonable to ask why there have not been more controlled clinical trials investigating the impact of reductions in dose intensity and the value of sustaining full dose intensity on long-term outcomes. Randomization between the dose and schedule shown to be effective in randomized controlled trials vs a regimen or schedule at considerably lower dose intensity that will likely be less effective raises important ethical concerns for both patients and oncologists. In addition, sample size or power considerations represent challenges to such trials as the number of patients needed in each arm of a trial to demonstrate the anticipated effect of a 10% to 25% reduction in relative dose intensity on patient survival number in the thousands (Figure 5). As a result, in most curative settings, large numbers of patients must be followed over many years costing millions or tens of millions of dollars.
Reduced Dose Intensity Chemotherapy in Practice
Concerns Related to Reduced Dose Intensity
Clearly, reduction in dose intensity relative to demonstrating efficacy in randomized controlled trials may diminish the potential for long-term disease control and survival in responsive and potentially curable malignancies. Although most oncologists find the evidence for maintaining dose intensity compelling, national practice pattern surveys conducted over the past few years continue to demonstrate that a large proportion of patients with potentially curable malignancies are undertreated. Chu and DeVita have concluded that such empiric reductions in dose intensity represent a major reason for treatment failure in patients with responsive malignancies in both the adjuvant and advanced disease situation.[23]
Practice Pattern Surveys of Delivered Chemotherapy Dose Intensity
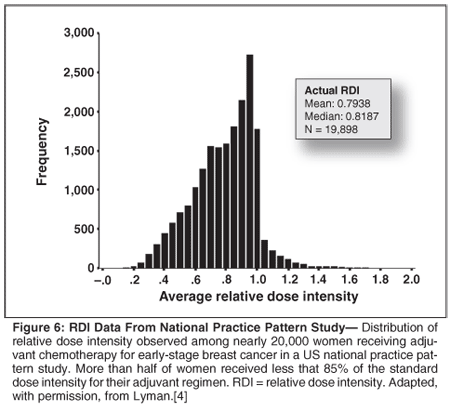
In a recent study of 20,000 women with early-stage breast cancer treated in 1,200 oncology practices, patients received an average relative dose intensity of 79% with more than half receiving less than 85% of standard dose intensity (Figure 6).[4] Nearly two-thirds of reductions in dose intensity were planned from the beginning of treatment while the remaining reductions were due to treatment-associated toxicity. In a similar study of over 4,500 patients with aggressive NHL receiving CHOP or CHOP-like chemotherapy, 53% and 48% received less than 85% of the standard dose intensity based on either treatment with six cycles or National Comprehensive Cancer Network guidelines, respectively.[5] Such undertreatment was more prevalent among elderly patients, those receiving certain regimens, and overweight or obese patients.
In a more recent survey between 1999 and 2002 in women with early-stage breast cancer, some 30% received less than 85% of standard relative dose intensity.[43] In multivariate analysis, significant independent predictors of reduced dose intensity included an episode of febrile neutropenia, greater age, body surface area > 2 m2, type of chemotherapy (particularly the use of anthracyclines-based regimens), and the number and type of comorbidities (particularly renal disease). After adjustment for these factors, primary prophylaxis with a colony-stimulating factor was associated with less reduction in dose intensity.
Reasons for Planned Reductions in Chemotherapy Dose Intensity
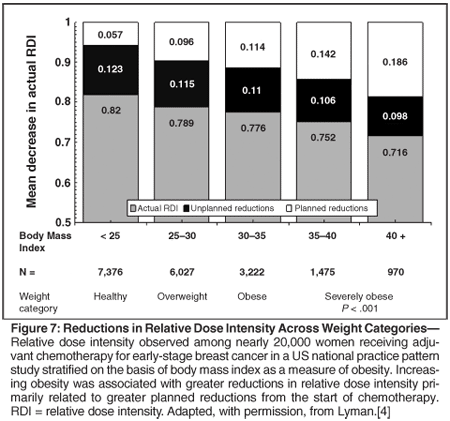
At least half of the reductions in chemotherapy dose intensity observed in national practice surveys are planned from the beginning of the treatment regimen. While all of the reasons for this intentional reduction in standard treatment doses and schedule are not clear, one frequent reason relates to the general incorporation of patient weight, usually in the form of body surface area, to calculate the dose to be administered. Multiple studies, including those discussed above, have demonstrated that reductions in standard dose intensity are frequent in overweight and obese patients (Figure 7).[4,44,45] Such modifications are frequent despite the consistent and prevailing evidence that obese individuals treated on the basis of actual body weight experience no greater hematologic toxicities than healthy weight individuals.[46]
Previous studies by the CALGB demonstrated that obese patients with early-stage breast cancer who received planned reductions in chemotherapy dose intensity were at increased risk for recurrence whereas those that received full dose intensity had no greater risk of toxicity and experienced recurrence rates comparable to healthy weight patients.[44] Other factors associated with planned reductions in chemotherapy toxicity include additional demographic factors such as age and race, clinical factors such as prior chemotherapy or radiation therapy, poor performance status, low blood counts, bone marrow involvement, and comorbidities such as liver, kidney, lung, and heart disease. Recent studies suggest that nonclinical factors such as socioeconomic status, education, and insurance status impact on planned dose intensity.[47]
Reasons for Unplanned Reductions in Chemotherapy Dose Intensity
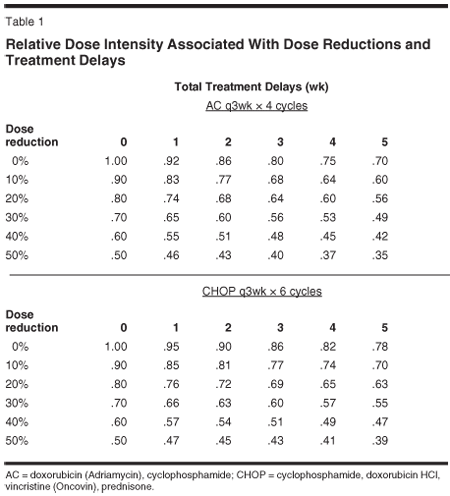
The single most important reason for reduced dose intensity during a course of chemotherapy is myelosuppression.[48] Inadequate recovery of neutrophils within the time of the treatment cycle often leads to treatment delays while severe or febrile neutropenia most often leads to dose reductions on subsequent cycles of chemotherapy. These may even combine in some patients resulting in early termination or alteration of the chemotherapy regimen. Obviously, there are some instances where reduced dose intensity is appropriate such as the noncurative setting where treatment is focused on the relief of symptoms and enhancement of patient quality of life.[6,7] Such situations often arise in the very elderly, frail or debilitated patient. Reduced dose intensity may also result from patient refusal or noncompliance. It is also known that the metabolism of drugs including chemotherapeutic agents vary among patients.[35] Overall, it is important to note how quick dose reductions and treatment delays may result in patients receiving major reductions in chemotherapy dose intensity (Table 1).
Colony-Stimulating Factor Use Can Sustain Dose Intensity
Perhaps the most important benefit from prophylactic CSF is the potential to sustain full dose intensity in responsive and potentially curable malignancies. Among the eight reporting randomized controlled trials of conventional dose chemotherapy in the recently updated meta-analysis, the average relative dose intensity among control and G-CSF patients was 88% (95% CI = 86%-90%) and 95% (95% CI = 93%-96%), respectively (P < .001) [49]. G-CSF-treated patients received 10% greater relative dose intensity than control patients among studies that included all ages, compared to a 5% increase in studies limited to elderly patients. Recent randomized controlled trials of G-CSF support among elderly patients with aggressive NHL receiving CHOP chemotherapy have demonstrated that patients receiving G-CSF achieved significantly greater chemotherapy relative dose intensity.[50,51] As noted previously, prospective studies of dose-dense regimens (every 2 weeks) requiring CSF support in both early-stage breast cancer and aggressive NHL have the potential for increasing the relative dose intensity of administered chemotherapy by 50%.[40,41]
Economic Considerations of Dose Intensity
Most economic evaluations of the myeloid growth factors have been based on cost minimization analyses where the tradeoff between the added cost of the growth factor and reduction in costs associated with preventing hospitalization for febrile neutropenia has been explored.[52,53] Recent studies have demonstrated reductions in early mortality including infection-specific mortality with G-CSF prophylaxis along with potential improvements in overall survival in the potentially curative setting.[50,54] This has generated recent studies of the cost-effectiveness of the hematopoietic growth factors based on their apparent ability to sustain or increase chemotherapy dose intensity.
An economic study in patients with small-cell lung cancer treated on a Medical Research Council trial (LU19) has recently been reported.[55] The observed prolongation increase in survival observed in the G-CSF supported arm of this study translated into an incremental cost-effectiveness ratio for this arm of approximately £25,000 per life-year extended. A recent randomized controlled trial of G-CSF support of CHOP chemotherapy in elderly patients with aggressive NHL demonstrated not only a significant reduction in the risk of febrile neutropenia and increase in delivered chemotherapy relative dose intensity but also a significant improvement in overall survival at 5 years.[50,56] An economic analysis of the cost-effectiveness of the G-CSF support for this patient population based on the reported trial results estimated an incremental cost-effectiveness ratio as low as $2,870 per life-year saved.
Chemotherapy Dose Intensity and Quality of Cancer Patient Care
While the quality of cancer patient care has always been the goal of every oncologist, it was not until the Institute of Medicine report on Ensuring Quality Cancer Care released in 1999 that this gained national attention.[57] This report concluded that there were many gaps in quality in cancer patient care and many quality indicators were either not useful or irrelevant. The report called for a national quality monitoring system and better quality measures. In response to this report, the American Society of Clinical Oncology and several other organizations began the National Initiative on Cancer Care Quality. A report of the findings of this initiative concluded that there are a number of areas in which the quality of oncology care needed improvement including the management of treatment-related side effects, optimizing chemotherapy dosing, and better documentation of key clinical information.[58]
As discussed in the article, there exists strong evidence in patients with responsive and potentially curable malignancies that reducing dose intensity by delaying treatments, lowering doses, premature discontinuation of treatment, or not treating increases the risk of disease recurrence and death from cancer. It is this author's contention that delivered chemotherapy dose intensity to patients with potentially curable malignancies should be considered a measure of quality of care that can be used as a benchmark of how well an oncology practice is doing. While others have called for such measures, the data have become increasingly compelling for chemotherapy dose intensity as one of the most important indicators of quality oncology care.[59,60]
While patient survival is perhaps the ultimate measure of the quality of cancer care, such data are seldom available and are confounded by the type and stage of disease referred to a practicing oncologist. Alternatively, process measures represent important surrogate measures that may impact on survival but represent more immediate indicators of the quality of cancer care. While appropriate measures of quality care in patients receiving cancer chemotherapy have been suggested, including adherence to guidelines, patient volume, and patient satisfaction delivered relative dose intensity where indicated and feasible should be considered a major quality indicator of optimal cancer care.
Disclosures:
Dr. Lyman has received research grant support from Amgen and also serves on their speakers bureau.
References:
1. Lyman GH, Kuderer NM: Epidemiology of febrile neutropenia. Support Cancer Ther 1:1-12, 2003.
2. Kuderer NM, Dale D, Crawford J, et al: The morbidity, mortality and cost of febrile neutropenia in cancer patients. Cancer 106: 2258-2266, 2006.
3. Caggiano V, Weiss RV, Rickert TS, et al: Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916-1924, 2005.
4. Lyman GH, Dale DC, Crawford J: Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol 21:4524-4531, 2003.
5. Lyman GH, Dale DC, Friedberg J, et al: Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: A nationwide study. J Clin Oncol 22:4302-4311, 2004.
6. National Comprehensive Cancer Network: Myeloid Growth Factors in Cancer Treatment Clinical Practice Guidelines in Oncology v. 2, 2006.
7. Smith TJ, Khatcheressian J, Lyman GH, et al: 2006 Update of Recommendations for the Use of White Blood Cell Growth Factors: An Evidence-Based Clinical Practice Guideline. J Clin Oncol 24:3187-3205, 2006.
8. Aapro MS, Cameron DA, Pettengell R, et al: European Organisation for Research and Treatment of Cancer (EORTC) Granulocyte Colony-Stimulating Factor (G-CSF) Guidelines Taskforce EORTC Guidelines for the use of Granulocyte Colony-Stimulating Factor in Adult Patients with Chemotherapy-Induced Febrile Neutropenia. Eur J Cancer 42:2433-2453, 2006.
9. Lyman GH: Pegfilgrastim: A granulocyte colony-stimulating factor with sustained duration of action. Exp Opin Biol Ther 5:1635-1646, 2005.
10. Bodey GP, Buckley M, Sathe YS, et al: Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328-340, 1966.
11. Crawford J, Dale DC, Lyman GH: Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 100:228-237, 2004.
12. Dale DC, McCarter GC, Crawford J, et al: Myelotoxicity and dose intensity of chemotherapy: Reporting practices from randomized clinical trials. J Natl Compr Canc Netw 1:440-454, 2003.
13. Hassett MJ, O'Malley AJ, Pakes JR, et al: Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 98:1108-1117, 2006.
14. Lyman GH, Morrison VA, Dale DC, et al: Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44:2069-2076, 2003.
15. Lyman GH, Delgado DJ: Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98:2402-2409, 2003.
16. Crawford J, Wolff D, Dale DC, et al: First-cycle risk of severe and febrile neutropenia in cancer patient receiving systemic chemotherapy: Results from a prospective nationwide study. Blood 104:11, 2004.
17. Lyman GH: Guidelines of the National Comprehensive Cancer Network on the Use of Myeloid Growth Factors with Cancer Chemotherapy: A Review of the Evidence. J Natl Compr Canc Netw 3:557-571, 2005.
18. Chouaid C, Bassinet L, Fuhrman C, et al: Routine use of granulocyte colony-stimulating factor is not cost-effective and does not increase patient comfort in the treatment of small-cell lung cancer: An analysis using a Markov model. J Clin Oncol 16:2700-2707, 1998.
19. Lyman GH, Kuderer NM: Economics of hematopoietic growth factors, in Morstyn G, Foote M, Lieschke GJ (eds): Cancer Drug Discovery and Development. Hematopoietic Growth Factors in Oncology: Basic Science and Clinical Therapeutics, pp 409-443. Totowa, NJ, Humana Press, 2004).
20. Cosler LE, Calhoun EA, Agboola O, et al: Effects of indirect and additional direct costs on the risk threshold for prophylaxis with colony-stimulating factors in patients at risk for severe neutropenia from cancer chemotherapy. Pharmacotherapy 24:488-494, 2004.
21. Longo DL, Duffey PL, DeVita VT Jr, et al: The calculations of actual or received dose intensity: A comparison of published methods. J Clin Oncol 9:2042-2051, 1991.
22. Hryniuk W, Levine MN: Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol 4:1162-1170, 1986.
23. Chu E, DeVita V: Principles of Medical Oncology, in Cancer: Principles and Practice of Oncology, 7th ed, pp 295-306. Philadelphia, Lippincott, 2005.
24. Skipper HE: Kinetics of mammary tumor cell growth and implications for therapy. Cancer 28:1479, 1971.
25. Hryniuk W, Bush H: The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol 2:1281-1288, 1984.
26. Meyer RM, Hryniuk WM, Goodyear MDE: The role of dose intensity in determining outcome in intermediate-grade non-Hodgkin's lymphoma. J Clin Oncol 9:339-347, 1991.
27. Bonadonna G, Valagussa P, Moliterni A, et al: Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up.
N Engl J Med 332:901-906, 1995.
28. Colleoni M, Li S, Gelber RD, et al: Relation between chemotherapy dose, estrogen receptor expression and body mass index. Lancet 366:1108-1110, 2005.
29. Lepage E, Gisselbrecht C, Haioun C, et al: Prognostic significance of received relative dose intensity in non-Hodgkin's lymphoma patients: Application to LNH-87 protocol. The GELA (Groupe d'Etude des Lymphomes de l'Adulte). Ann Oncol 4:651-656, 1993.
30. Epelbaum R, Faraggi D, Ben Arie Y, et al: Survival of diffuse large cell lymphoma. A multivariate analysis including dose intensity variables. Cancer 66:1124-1129, 1990.
31. Lee K-W, Kim DY, Yun T et al: Doxorubicin-based chemothreapy for diffuse large B-cell lymphoma in elderly patients: Comparison of treatment outcomes between young and elderly patients and the significance of doxorubicin dosage. Cancer 98:2651-2656, 2003.
32. Kwak LW, Halpern J, Olshen RA, et al: Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J Clin Oncol 8:963-977, 1990.
33. Hershman DL, Wang X, McBride R, et al: Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat 99:313-321, 2006.
34. Mayers C, Panzarella T, Tannock IF: Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer 91:2246-2257, 2001.
35. Di Maio M, Gridelli C, Gallo C, et al: Chemotherapy-induced neutropenia and treatment efficacy in advance non-small-cell lung cancer: A pooled analysis of three randomised trials. Lancet 6:669-677, 2005.
36. Early Breast Cancer Trialists' Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet 365:1687-1717, 2005.
37. Budman DR, Berry DA, Cirrincione CT, et al: Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B.
J Natl Cancer Inst 90:1205-1211, 1998.
38. Bonneterre J, Roche H, Kerbrat P, et al: Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol 23:2686-2693, 2005.
39. Ardizzoni A, Favaretto A, Boni L, et al: Platinum-etoposide chemotherapy in elderly patients with small-cell lung cancer: Results of a randomized multicenter phase II study assessing attenuated-dose or full-dose with lenograstim prophylaxis-A Forza Operativa Nazionale Italiana Carcinoma Polmonare and Gruppo Studio Tumori Polmonari Veneto (FONICAP-GSTPV) study. J Clin Oncol 23:569-575, 2005.
40. Citron ML, Berry DA, Cirrincione C, et al: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431-1439, 2003.
41. Pfreundschuh M, Trümper L, Kloess M, et al: 2-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: Results of the NHL-B2 trial of the DSHNHL. Blood 104:634-641, 2004.
42. Norton L, Simon R: Tumor size, sensitivity to therapy and design of treatment schedules. Cancer Treat Rep 61:1307-1317, 1977.
43. Shayne M, Crawford J, Dale DC, et al: Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat 2006 (e-pub ahead of print).
44. Rosner GL, Hargis JB, Hollis DR, et al: Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: Results from Cancer and Leukemia Group B Study 8541. J Clin Oncol 14:3000-3008, 1996.
45. Madarnas Y, Sawka CA, Franssen E, et al: Are medical oncologists biased in their treatment of the large women with breast cancer? Breast Cancer Res Treat 66:123-133, 2001.
46. Griggs JJ, Sorbero MES, Lyman GH: Undertreatment of obese women receiving breast cancer chemotherapy. Arch Int Med 165:1267-1273, 2005.
47. Griggs JJ, Culakova E, Sorbero MES, et al: The effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol 2006 (in press).
48. Lyman GH, Lyman CH, Agboola O: Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10:427-437, 2005.
49. Lyman GH, Kuderer NM, Djulbegovic B: Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: A meta-analysis. Am J Med 112:406-411, 2002.
50. Osby E, Hagberg H, Kvaloy S, et al: CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: Results of a Nordic Lymphoma Group randomized trial. Blood 101:3840-3848, 2003.
51. Doorduijn JK, van der Holt B, van Imhoff GW, et al: CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin's lymphoma. J Clin Oncol 21:3041-3050, 2003.
52. Lyman GH, Lyman CG, Sanderson RA, et al: Decision analysis of hematopoietic growth factor use in patients receiving cancer chemotherapy. J Natl Cancer Inst 85:488-493, 1993.
53. Lyman GH, Kuderer N, Greene J, et al: The economics of febrile neutropenia: Implications for the use of colony-stimulating factors. Eur J Cancer 34:1857-1864, 1998.
54. Kuderer NM, Crawford J, Dale DC, et al: Meta-analysis of prophylactic granulocyte colony-stimulating factor in cancer patients receiving chemotherapy (abstract 8117). J Clin Oncol 23(suppl 16S):758s, 2005.
55. Bojke L, Sculpher M, Stephens R, et al: Cost effectiveness of increasing the dose intensity of chemotherapy with granulocyte colony-stimulating factor in small-cell lung cancer. Pharmacoeconomics 24:443-452, 2006.
56. Lyman GH, Eldar Lissai A, Cosler L: Cost-effectiveness analysis of G-CSF support elderly patients with aggressive non-Hodgkin's lymphoma receiving CHOP. Value in Health A107, 2006.
57. Hewitt M, Simone JV (eds): Institute of Medicine: Ensuring Quality Cancer Care. Washington, DC, National Academy Press, 1999.
58. Malin JL, Schneider EC, Epstein AM, et al: Results of the National Initiative for Cancer Care Quality: How can we improve the quality of cancer care in the United Sates? J Clin Oncol 24:626-634, 2006.
59. Morrow T, Siegel M, Boone S, et al: Chemotherapy dose intensity determination as a quality of care measure for managed care organizations in the treatment of early-stage breast cancer. Am J Med Qual 17:218-224, 2002.
60. Ottevanger PB, De Mulder PHM: The quality of chemotherapy and its quality assurance. Eur J Surg Oncol 31:656-666, 2005.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.