Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most challenging and complex complications of cancer chemotherapy.
ABSTRACT: An important role of the oncology nurse is to monitor and continually assess the patient for chemotherapy-induced peripheral neuropathy, which is a complication occurring in up to 90% of patients treated with neurotoxic chemotherapy agents. Severity and incidence depend on specifics of treatment and patient comorbidities and lifestyle factors. Symptoms vary widely and will adversely affect quality of life, but early intervention can prevent exacerbation and may restore neurological function. The case study discussed in this article illustrates the manifestations of neuropathy affecting sensory, autonomic, and motor function, and shows how both physical and mental health therapies can be effective. To support the cancer survivor during and after chemotherapy, the oncology nurse will manage neurological impairment by examination, grading systems, pharmacological and nonpharmacological approaches, and patient education and referrals.
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most challenging and complex complications of cancer chemotherapy. Peripheral neuropathy is a disturbance of function or pathological change in a nerve or nerves,[1] and CIPN generally is diffuse and bilateral, resulting from systemic toxicity to nerves. Several classes of chemotherapeutic drugs cause peripheral neuropathy, including the plant alkaloids (vincristine and vinblastine), taxanes (paclitaxel and docetaxel [Taxotere]), platinum-based compounds (cisplatin, carboplatin, and oxaliplatin [Eloxatin]), and other drugs, such as thalidomide.
The incidence and severity of CIPN vary considerably for each peripherally neurotoxic agent when administered alone or in combination, but for vincristine, cisplatin, oxaliplatin, and paclitaxel, estimates for the occurrence of CIPN are as high as 70% to 90%.[2–6] As many as 60% of patients receiving docetaxel and 40% of those treated with carboplatin develop CIPN.[3,7] The development of both short- and long-term CIPN is highly dependent on factors such as age, single dose intensity, cumulative dose, duration of therapy, combinations of neurotoxic agents, coexisting neuropathies (for example, diabetic neuropathy), genetic susceptibility, and alcohol abuse.[8–14]
Exact mechanisms for CIPN are not clearly understood; however, studies indicate that it is related to axonal damage of peripheral nerves, causing dysfunctional effects in primary afferent fibers that give rise to abnormal impulse transmission, nerve hyperexcitability, spontaneous or ectopic discharge from nerves, and pain.[15–18] Neurotoxic effects of chemotherapy target the neuronal cell body, the axonal transport system, the myelin sheath, and glial support structures of peripheral nerves.[19]
The onset, severity, characteristics, and duration of clinical manifestations of CIPN are highly variable. Typically, CIPN is characterized by a glove-and-stocking distribution in the hands and feet with sensory loss or hypersensitivity, and in some cases motor and autonomic dysfunction. Symptoms include paresthesia (an abnormal sensation such as numbness or tingling, spontaneous or evoked, and not unpleasant), dysesthesia (an unpleasant abnormal sensation, spontaneous or evoked), allodynia (pain from stimuli that are not typically painful, such as touch), hyperalgesia (exaggerated pain in response to stimuli that are typically painful), hypoalgesia (diminished pain response to a typically painful stimulus), or pain that is burning, shooting, or electric-shock–like.[1]
Patients also may experience a loss of temperature sensation, of proprioception, and of vibratory sensing; weakness in their extremities; or ataxia. Sensory and motor disturbances are often a function of specific chemotherapy agents. For example, paclitaxel can induce sensory impairment and pain, whereas vincristine may produce a sensorimotor neuropathy and motor dysfunction such as foot drop.[20] Motor and autonomic nervous system involvement is typically seen with vincristine and platinum-based compounds.
Several grading systems are used to classify the severity of CIPN. Among the most common are the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE) and the Eastern Cooperative Oncology Group Neuropathy Scale. Both measure sensory and motor abnormalities on a grading scale of 0 (absent) to 4 (severe) based on findings from physical examination and patient reports.[21–23]
The Total Neuropathy Scale (TNS) is less frequently used in clinical practice, but is much more comprehensive. The TNS measures sensory and motor symptoms, pin sensibility, vibration sensibility, and reflexes, and it incorporates information from quantitative sensory testing (QST) and electromyography to quantify autonomic symptoms, thermal and vibration thresholds, and nerve signal amplitudes.[24] The TNS demonstrates greater sensitivity to sensory and motor problems, and more precisely quantifies changes over time.[24] More information about these and other scales to assess peripheral neuropathy is provided online by the Oncology Nursing Society.[25]
The incidence and severity of CIPN related to specific cancer chemotherapy regimens have been well studied; however, less is known about the clinical course of CIPN and its long-lasting effects on cancer survivors’ daily function and quality of life (QoL).[26] CIPN can be a persistent and debilitating problem. Follow-up care for cancer survivors must include ongoing monitoring for CIPN, especially if patients experience this problem during therapy. Early and aggressive treatment with analgesics and supportive interventions (eg, physical and occupational therapy) may help to prevent worsening of painful and debilitating CIPN and long-term QoL consequences.
Patient Overview
The patient, “JB,” is a 38-year-old premenopausal female with insulin-controlled type-2 diabetes. She was diagnosed with invasive ductal carcinoma of the right breast in 2007. Based on the American Joint Committee on Cancer TNM staging system, her tumor was staged at T (tumor size), T2, 2.5 cm; N (nodal status), spread to lymph nodes, 2 of 5; and M (metastases), M0 (no evidence of distant disease). Receptor assays indicated that her tumor was estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal growth factor receptor 2 (HER2) positive
She underwent a radical right mastectomy followed by reconstructive surgery with a TRAM (transverse rectus abdominus myocutaneous) flap. After recovering from surgery, she was treated with a chemotherapy regimen that included four cycles of dose-dense doxorubicin at 60 mg/m2 and cyclophosphamide at 600 mg/m2 every 2 weeks, followed by weekly paclitaxel at 80 mg/m2 for 12 weeks and trastuzumab (Herceptin) given as a 4 mg/kg loading dose in the first week and then at 2 mg/kg weekly for 12 weeks. Therapy with tamoxifen was initiated, and she completed an adjuvant regimen with trastuzumab at 6 mg/kg every 3 weeks for 1 year.
With her eleventh dose of paclitaxel, JB had evidence of a Grade 3 sensory neuropathy by the NCI CTCAE grading system (sensory alteration or paresthesia interfering with activities of daily living) and Grade 1 motor neuropathy (asymptomatic, with weakness detected on examination or by testing only). A decision was made to continue therapy with the last course of treatment.
JB now presents to the oncology clinic for follow-up, complaining that over the past 8 months she has had difficulty walking. Her oncology nurse obtains specific information about how JB has been doing over the last 6 months. JB reports feeling as though her balance is off and describes a constant burning discomfort in her hands and feet. Occasionally, she experiences sharp, shooting, and electric-shock–like pains in her feet. She also says that she has had constant burning sensations in her hands and feet since completing her chemotherapy.
JB neglected to report these symptoms during prior follow-up visits because she hoped that these sensory and motor experiences would resolve after therapy. This did not happen, and her symptoms have worsened to the point that it is uncomfortable for her to wear shoes and walk. She states that it is often difficult to start walking when she has been seated for extended periods of time, and her legs feel weak and heavy. She is also disturbed by “pins and needles” sensations in her hands, and finds herself dropping things.
At times, she has difficulty with fine motor movements, and touch causes pain in her fingertips, interfering with her ability to write, use a computer at work, and correspond on her Blackberry. JB tells her oncology nurse that she is frightened, frustrated, and depressed over her current situation. She admits that she cries frequently, is unable to sleep, and has no energy. JB believes that her level of function has declined and these distressing symptoms have significantly affected her quality of life and ability to work; however, she is still able to care for her family.
She denies any symptoms of fever, chills, headache, cough, chest pain, nausea, vomiting, or diarrhea, and has no history of trauma to her back or extremities. Her health history is significant for type 2 diabetes diagnosed 8 years ago, during the last trimester of her pregnancy. Health records from her primary care physician indicate that she has not achieved adequate glycemic control, with glycated hemoglobin levels (A1C) in the range of 7.8% to 8.6% in the past 6 months. Also, 13 years ago, she had Lyme disease, which was treated with a course of doxycycline. She denies alcohol or illicit drug use but still continues to smoke about 1 pack of cigarettes per week. She lives with her husband and 8-year-old son, and is currently on medical leave of absence from her job as a paralegal.
Nursing Management
It is important that oncology nurses recognize the widespread problem of CIPN in patients receiving peripherally neurotoxic chemotherapy agents. Moreover, it is critical that oncology nurses continually assess patients for evidence of CIPN during and after cancer treatment. It is not uncommon for CIPN to worsen after treatment, especially in the presence of coexisting conditions such as diabetic neuropathy.
Oncology nurses must be familiar with evidence regarding the effectiveness of pharmacological and nonpharmacological approaches to managing CIPN. Visovsky et al[27] have evaluated and summarized the evidence for CIPN treatment strategies in “Putting Evidence Into Practice.” This resource provides useful information to guide the care of patients and evidence to support practice.
Nursing assessment of CIPN begins with a comprehensive approach to collecting subjective information from the patient, as well as assimilating clinical findings from physical examinations or neurological tests. Oncology nurses should evaluate sensory symptoms, motor symptoms, and autonomic symptoms (constipation, urinary retention, sexual dysfunction, blood pressure alterations).[27]
Standards of care for patients at risk for or with CIPN are based on a thorough assessment of sensory symptoms such as pain; diminished or heightened sensations; cutaneous hypersensitivity of the hands and feet (allodynia or hyperalgesia); the ability to discriminate sensations of cold, sharp, and dull; and absence of the ability to detect stimuli (numbness), response to vibration, and positioning of distal areas such as the toes and fingers. The character and quality of pain are evaluated by word descriptors such as “sharp,” “dull,” “burning,” “shooting,” or “electric-shock–like.”
Patients should have a thorough neurological examination, including assessment of gait, motor and sensory functions, deep tendon reflexes, and nerve conduction tests. Grading systems for peripheral neuropathy previously discussed can be used to categorize the degree of neurological impairments. It is important to recognize that most such systems are just global assessments of peripheral neuropathy and lack the sensitivity to precisely quantify the degree of neurological dysfunction. Nonetheless, these systems are particularly useful in documenting early CIPN and assessing changes over time.
Patients with more severe peripheral neuropathies (Grade 3 or 4) can be advised of the consequences of continuing treatment with neurotoxic chemotherapeutic agents, and dose adjustments for chemotherapy can be made based on these findings. In some cases, more specific and reliable tests can be performed, such as QST (quantitative sensory testing), a noninvasive assessment and quantification of sensory nerve function (ie, tactile and thermal thresholds) in patients with symptoms. Generally, QST is performed by a neurologist or pain specialist.
JB has described her difficulty performing specific activities and tasks. She also has reported abnormal sensations (dysesthesias) in her hands and feet, and the quality and character of her pain. The oncology nurse asks her to rate her levels of pain using a numeric (0 to 10) rating scale. JB says that the average pain intensity in her hands and feet ranges from 4 to 5, and her worst pain levels are often at 6 or 7.
TABLE 1
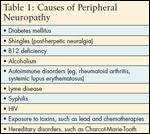
Causes of Peripheral Neuropathy
A nurse practitioner performs a neurologic examination on JB. There is a significant decrease in detection of pain and temperature in the plantar aspects of her feet, bilaterally. There is diminished or absent sense of discrimination between sharp (pinprick) and dull stimuli on the hand (dorsal and palmar) and foot (dorsal and plantar) surfaces, as well as medial and lateral areas. She has full range of motion, but weakness in muscle strength is noted in just her lower extremities. Deep tendon reflexes are normal, with the exception of the Achilles reflexes, which are absent bilaterally. Proprioception is reduced and vibration sense is diminished in great toes bilaterally. Her gait is slightly unsteady. The rest of her physical examination is unremarkable. JB is diagnosed with a NCI CTCAE Grade 3 sensory neuropathy, but has progressed to a Grade 2 motor neuropathy (symptomatic weakness, interfering with function, but not interfering with activities of daily living).
TABLE 2
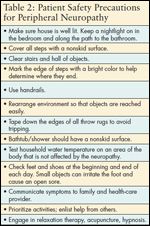
Patient Safety Precautions for Peripheral Neuropathy
Upon reviewing JB’s history for risks of peripheral neuropathy, it is important to consider her cancer treatment and health history. This patient completed her last course of chemotherapy 13 months ago. The risk of Grade 2, 3, and 4 CIPN is increased with weekly paclitaxel vs every-3-week dosing.[28] Coexisting diabetes in patients treated with paclitaxel or other neurotoxic chemotherapeutic agents also increases the likelihood for developing CIPN.[4,29] Lyme disease, if untreated, can result in peripheral neuropathy, and nicotine from cigarette smoking constricts blood vessels that supply nutrients to the peripheral nerves (see Table 1). She is referred to a neurologist for QST, which reveals sensory and motor neuropathy. JB is most likely experiencing long-term CIPN related to treatment with paclitaxel that is complicated by a peripheral neuropathy from her poorly controlled diabetes. The nurse practitioner consults with JB’s medical oncologist, and they agree to initiate therapy with gabapentin at 200 mg twice a day for 5 days, then 200 mg three times a day for 5 days, followed by an increase to 300 mg three times a day. The effectiveness of gabapentin for CIPN has been documented,[30] but in one study it was not effective.[31] Gabapentin is beneficial, however, for treatment of diabetic neuropathy.[32] If JB’s symptoms of depression persist after titrating gabapentin to an effective dose for pain relief, which can be up to 3,600 mg per day, then duloxetine (Cymbalta) at 60 mg per day could be added to her treatment regimen. Duloxetine is also useful not only for depression, but also for neuropathic pain from diabetic neuropathy.[32]
TABLE 3
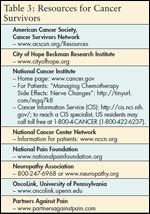
Resources for Cancer Survivors
Patient education is an important part of nursing care for patients with CIPN. Several comprehensive reviews outline the responsibility of all healthcare professionals caring for cancer patients and survivors to teach these individuals how to self-manage their care when experiencing neurologic damage from cancer treatment.[3,26,27,33] Simple measures can and should be taken to protect hands and feet from injury. These include wearing oven mitts when cooking; gloves when gardening; properly fitting shoes; protective wear in the cold; assessing water temperature to prevent exposure to extreme cold or heat; and preventing falls by keeping rooms well lit, clearing walkways, and using nonskid mats in showers and bathtubs (see Table 2).[34]
It is important for nurses to support patients and their families by helping them adapt to temporary or perhaps lifelong changes in daily routines. CIPN can cause patients to lose their balance or have difficulty with motor skills such as picking things up, buttoning clothes, opening jars, or inserting keys in keyholes. Fatigue and depression are also commonly associated with CIPN. Patients and families should report any sensory, motor, or autonomic symptoms to their healthcare professionals. Referrals to local and national resources can be helpful to many cancer survivors with CIPN (see Table 3). For example, the Neuropathy Association (reachable at 800-247-6968 or online at www.neuropathy.org) is a national organization that works toward increasing public awareness of the problem and promoting development of better therapies for this condition. It offers information, self-help resources, and support group networks to patients with peripheral neuropathy.
Discussion
To date, there is no compelling evidence for therapeutic interventions to effectively and reliably prevent CIPN. Many prevention strategies have been studied, but in large part these have not proven to be very effective. Novel pharmacologic agents such as neuroprotective compounds (amifostine or WR-2721 [Ethyol]), glutamine and L-carnitine (amino acids), or glutathione (an antioxidant and product of glutamine metabolism), and/or neurotrophic factors (eg, nerve growth factor) are of limited benefit in preventing CIPN or reducing its severity.[35]
Glutamine, which acts as a substrate for dividing cells, may help to prevent or minimize the severity of peripheral neuropathy related to paclitaxel-induced neurotoxicity.[36] In a recent systematic review of neuropathy prevention interventions for cisplatin and related compounds, investigators found that there is insufficient evidence to support the role of neuroprotectants in preventing or limiting CIPN and more research is needed to establish their efficacy and safety.[37]
Vitamin E supplementation in doses of 600 mg daily has shown the greatest promise for prophylaxis of CIPN with cisplatin-based compounds and paclitaxel in small, well-designed clinical trials.[38–40] New evidence has emerged to favor the use of intravenous calcium and magnesium therapy, which can attenuate the development of oxaliplatin-induced CIPN. In doses of 1 g each, it is hypothesized that increasing concentrations of extracellular calcium facilitates closing of sodium channels, which has the potential to decrease the observed oxaliplatin-induced hyperexcitability of peripheral neurons.[41] Extensive reviews have summarized research thus far on use of novel agents in the prevention and treatment of CIPN.[27,42]
Effective treatment approaches for painful CIPN include both pharmacological and nonpharmacological interventions. Analgesic therapy with anticonvulsants (gabapentin, pregabalin [Lyrica], or lamotrigine), tricyclic antidepressants (amitriptyline, nortriptyline, or desipramine), serotonin-norepinephrine reuptake inhibitor antidepressants (venlafaxine and duloxetine), and opioids are effective in the treatment of neuropathic pain syndromes including neuropathies.[43–46] Some patients get relief from topical analgesics, such as capsaicin or a lidocaine patch.
Nonpharmacological and alternative therapies have been used to treat CIPN. In a clinical case report, two patients benefited from an implanted spinal cord stimulator that alleviated pain, increased leg flexibility, and led to improvements in sensory threshold detection.[47] Nutritional supplements such as evening primrose oil and alpha-lipoic acid, and topical analgesics including capsaicin and local anesthetics, can be effective in the treatment of neuropathies.[48] Exercise and occupational therapy can restore function in extremities, but studies of their effectiveness have mostly been done in the early treatment phase. Exercise can also enhance balance, strength, and safety, and braces can be used to support weak muscles. Occupational therapy may improve fine motor coordination and therapists can provide assistive devices and help to adapt home environments to address safety issues and practical concerns related to neuropathy.
Conclusion
JB was started on a gabapentin titration schedule. She was referred to an occupational therapist to assist her with fine motor skills and a physical therapist for leg strengthening exercises 3 days per week. A home health nurse referral was initiated to evaluate the safety of her home and teach her safety precautions to prevent injury. After 3 months of therapy with gabapentin at 400 mg three times a day and intensive occupational and physical therapy, JB returns to the oncology clinic for a follow-up visit.
Her symptoms and function have improved to the point where she can walk a mile a day, do housework, and type on her computer. She regularly attends a Neuropathy Association Support Group close to her home one evening each month, and takes advantage of the association’s online resource as well as information and support from the National Pain Foundation and American Pain Foundation. She has also joined the American Cancer Society Cancer Survivors Network and says she no longer feels alone. JB reports better quality of sleep, being less depressed, and socializing more with family and friends. She is optimistic that with the help of her family and friends and local and national support networks she can return to work.
Clearly, oncology nurses assume an important role in the education and clinical support of cancer patients and survivors experiencing peripheral neuropathy. Oncology nurses can provide effective interventions and practical guidance to greatly improve patient safety and quality of life.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
Financial Disclosure: The author has no significant fi nancial interest or other relationshipwith the manufacturers of any productsor providers of any service mentioned inthis article.
References
1. Merskey H, Bogduk N (eds): Classification of Chronic Pain, 2nd ed, Part III. IASP Task Force on Taxonomy, IASP Press, Seattle, 1994, pp 209â214.
2. Polomano RC, Bennett GJ: Chemotherapy-evoked painful peripheral neuropathy. Pain Med 2(1):8â14, 2001.
3. Armstrong T, Almadrones L, Gilbert MR: Chemotherapy induced peripheral neuropathy. Oncol Nurs Forum 32(2):305â311, 2005.
4. Hausheer FH, Schilsky RL, Bain S, et al: Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 33(1):15â49, 2006.
5. Wilkes G: Peripheral neuropathy related to chemotherapy. Semin Oncol Nurs 23(3):162â173, 2007.
6. Argyriou AA, Polychronopoulos P, Iconomou G, et al: A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev 34(4):368â377, 2008.
7. Wampler MA, Hamolsky D, Hamel K, et al: Case report: Painful peripheral neuropathy following treatment with docetaxel for breast cancer. Clin J Oncol Nurs 9(2):189â193, 2005.
8. Visovsky C: Chemotherapy-induced peripheral neuropathy. Cancer Invest 21(3):439â451, 2003.
9. Repetto L: Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol 1(4 Suppl 2):18â24, 2003.
10. Dropcho EJ: Neurotoxicity of cancer chemotherapy. Semin Neurol 24(4):419â426, 2004.
11. Kannarkat G, Lasher EE, Schiff D: Neurologic complications of chemotherapy agents. Curr Opin Neurol 20(6):719â725, 2007.
12. Argyriou AA, Koltzenburg M, Polychronopoulos P, et al: Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 66(3):218â228, 2008.
13. McWhinney SR, Goldberg RM, McLeod HL: Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 8(1):10â16, 2009.
14. Nurgalieva Z, Xia R, Liu CC, et al: Risk of chemotherapy-induced peripheral neuropathy in large population-based cohorts of elderly patients with breast, ovarian, and lung cancer. Am J Ther May 15, 2009 [Epub ahead of print].
15. Cata JP, Weng HR, Lee BN, et al: Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anesthesiol 72(3):151â169, 2006.
16. Dougherty PM, Cata JP, Burton AW, et al: Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage 33(2):166â179, 2007.
17. Xiao WH, Bennett GJ: Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain 135(3):262â270, 2008.
18. Park SB, Krishnan AV, Lin CS, et al: Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem 15(29):3081â3094, 2008.
19. Malik B, Stillman M: Chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep 12(3):165â174, 2008.
20. Dropcho EJ: Neurotoxicity of cancer chemotherapy. Semin Neurol 24(4):419â426, 2004.
21. Common Terminology Criteria for Adverse Events (CTCAE). US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Version 4, May 2009. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev4.pdf. Accessed on June 1, 2009.
22. Postma T, Heimans J: Grading of chemotherapy-induced peripheral neuropathy. Ann Oncol 11(5):509â513, 2000.
23. Oken MM, Creech RH, Tormey DC, et al: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649â655, 1982.
24. Cavaletti G, Frigeni B, Lanzani F, et al: The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute Common Toxicity Scale.
J Peripher Nerv Syst 12(3):210â215, 2007.
25. Oncology Nursing Society: Measuring Oncology Nursing-Sensitive Patient Outcomes: Evidence-based Summary. Chemotherapy-induced Peripheral Neuropathy. Available at: http://www.ons.org/outcomes/Clinical/pdf/NeuropathySummary.pdf. Accessed on June 2, 2009.
26. Polomano RC, Farrar JT: Pain and neuropathy in cancer survivors. Surgery, radiation, and chemotherapy can cause pain: Research could improve its detection and treatment. Am J Nurs 106(3 Suppl):39â47, 2006.
27. Visovsky C, Collins M, Abbott L, et al: Putting evidence into practice: Evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs 11(6):901â913, 2007.
28. Vahdat L: Choosing a taxane for adjuvant treatment of breast cancer: More than a flip of the coin? Natl Clin Pract Oncol 5(10):570â571, 2008.
29. Mielke S, Mross K, Gerds TA, et al: Comparative neurotoxicity of weekly non-break paclitaxel infusions over 1 versus 3 h. Anticancer Drugs 14(10):785â792, 2003.
30. Tsavaris N, Kopterides P, Kosmas C, et al: Gabapentin monotherapy for the treatment of chemotherapy-induced neuropathic pain: A pilot study. Pain Med 9(8):1209â1216, 2008.
31. Rao RD, Michalak JC, Sloan JA, et al: North Central Cancer Treatment Group: Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110(9):2110â2118, 2007.
32. Quilici S, Chancellor J, Löthgren M, et al: Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol (online) 9:6, 2009.
33. Arnstein P: Chronic neuropathic pain: Issues in patient education. Pain Manag Nurs 5(4 Suppl 1):34â41, 2004.
34. Abramson Cancer Center of the University of Pennsylvania, OncoLink: Managing Symptoms: Peripheral Neuropathy (Nerve Damage). Available at: http://www.oncolink.com/coping/article.cfm?c=5&s=27&ss=52&id=577. Accessed on May 29, 2009.
35. Cavaletti G, Zanna C: Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity. Eur J Cancer 38(14):1832â1837, 2002.
36. Savarese DM, Savy G, Vahdat L, et al: Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev 29(6):501â513, 2003.
37. Albers J, Chaudhry V, Cavaletti G, et al: Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev Jan 24(1):CD005228, 2007.
38. Argyriou AA, Chroni E, Koutras A, et al: Vitamin E for prophylaxis against chemotherapy-induced neuropathy: A randomized controlled trial. Neurology 64(1):26â31, 2005.
39. Argyriou AA, Chroni E, Koutras A, et al: A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: Final results. Support Care Cancer 14(11):1134â1140, 2006.
40. Argyriou AA, Chroni E, Koutras A, et al: Preventing paclitaxel-induced peripheral neuropathy: A phase II trial of vitamin E supplementation. J Pain Symptom Manage 32(3):237â244, 2006.
41. Armstrong CM, Cota G: Calcium block of Na+ channels and its effect on closing rate. Proc Natl Acad Sci USA 96(7):4154â4157, 1999.
42. Wolf S, Barton D, Kottschade L, et al: Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer 44(11):1507â1515, 2008.
43. Cavaletti G, Zanna C: Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity. Eur J Cancer 38(14):1832â1837, 2002.
44. Ocean AJ, Vahdat LT: Chemotherapy-induced peripheral neuropathy: Pathogenesis and emerging therapies. Support Care Cancer 12(9):619â625, 2004.
45. Gordon DB, Love G: Pharmacological management of neuropathic pain. Pain Manag Nurs 5(Suppl 1):19â33, 2005.
46. Dworkin RH, O’Connor AB, Backonja M, et al: Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 132(3):237â251, 2007.
47. Cata JP, Cordella JV, Burton AW, et al: Spinal cord stimulation relieves chemotherapy-induced pain: A clinical case report. J Pain Symptom Manage 27(1):72â78, 2004.
48. Rock E, DeMichele A: Nutritional approaches to late toxicities of adjuvant chemotherapy in breast cancer survivors. J Nutr 133(11 Suppl 1):3785Sâ3793S, 2003.