Before CNS Benefits of Erythropoietic Agents Can Be Realized, Transport Mechanisms Must Be Understood
THOUSAND OAKS, California-How do endogenous and exogenous erythropoietin (EPO) directly and indirectly affect the central nervous system (CNS)? How can erythropoietic agents penetrate the blood-brain/blood-cerebrospinal fluid (CSF) barrier so that their potential neuroprotective benefits can be realized?
THOUSAND OAKS, CaliforniaHow do endogenous and exogenous erythropoietin (EPO) directly and indirectly affect the central nervous system (CNS)? How can erythropoietic agents penetrate the blood-brain/blood-cerebrospinal fluid (CSF) barrier so that their potential neuroprotective benefits can be realized?
While these questions are still being investigated, some interim findings were presented by Nelson L’ntshotsholé Jumbe, PhD, research scientist, pharmacokinetics and drug metabolism, at Amgen in Thousand Oaks, California.
"Astrocytes and neurons have been shown to produce erythropoietin and to express erythropoetic receptors," Dr. Jumbe said. "There’s also evidence that CNS EPO mRNA, like systemic mRNA, is hypoxia-inducible."
A learning test study with gerbils showed that brain activity requires free-floating erythropoietin in the interstitial space, Dr. Jumbe reported. In animals with 3-minute forebrain ischemia, exogenous erythropoietin had a neuroprotective effect at doses ranging from 0.5 to 25 units/day over 7 days, administered as continuous infusion and started 8 to 24 hours before the ischemic event. The effect was not concentration-dependent. "It was either an on or off mechanism; either it occurred or it didn’t," Dr. Jumbe said. He noted that no activity occurred at higher EPO concentrations.
Soluble erythropoietic receptors reversed the effects of erythropoietic therapy by binding with free-floating EPO. "This shows that you need free-floating EPO in the interstitial space to get the neurons that are destined for degeneration to survive...The same result was not seen when you used denatured soluble erythropoietin receptor," Dr. Jumbe said. "What the authors hypothesize from these data is that brain EPO is pivotal in maintenance and recovery of neuronal viability."
Central and Peripheral EPO
Dr. Jumbe cited work by Chikuma et al (1998) that found no direct link between central and peripheral EPO. During continuous hypoxia over 24 hours, EPO mRNA was induced in the kidney and cerebrum of mice. In the kidney, "after 4 hours, the message was downregulated and the serum levels also followed suit," Dr. Jumbe reported. "However, in the CNS, the total message was upregulated throughout the 24-hour period. This shows that the CNS and systemic messages are actually not linked at all and that these systems behave quite differently. This might be due to the different roles that EPO plays in different regions," he explained.
This study was the first to suggest that systematically administered erythropoietic therapy could cross the blood-brain barrier and the authors hypothesized that transport of erythropoietin was mediated by EPO receptors. There is also a bone marrow barrier, Dr. Jumbe noted, and if EPO gets into the bone marrow, "there might be a transfer process that also regulates access to the brain. This transport mechanism has not been investigated directly but commands interest,’’ he said.
Barrier Dysfunction
In a case study, 6,000 units of exogenous erythropoietin (about 50 units/kg, a low dose) administered IV did not change CSF EPO levels. Changes did occur in carbon dioxide and sodium levels in the CSF and in pH. "This led the authors to hypothesize that the modification of CNS by systemic erythropoietic therapy occurs via an acid balance change and that there are possible indirect effects manifested in the brain following systemic administration," Dr. Jumbe said.
He pointed to strong evidence for transport of exogenous erythropoietin across impaired blood-barrier and blood-CSF barriers, but disparate findings for access across intact barriers. Still to be determined is "whether the indirect action of systemic drugs on CNS is via changed blood viscosity, iron levels, and better oxygenation" and if these indirect effects are actually responsible for better patient outcome.
Pilot Permeability Studies
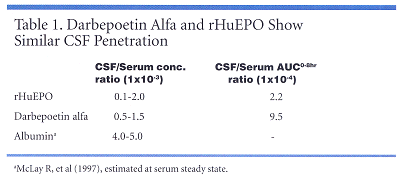
A pilot study with rats found that darbepoetin alfa (Aranesp) and recombinant human erythropoietin showed similar permeation across the blood-CSF barriers-despite differences in size, mass, and charge. Both agents are measurable in rat CSF up to 8 hours after high-dose IV administration and the calculated penetration is of the same order as reported values for albumin (see Table 1). ‘‘None of our current data provide evidence of a saturable transport mechanism of either darbepoetin alfa or rHuEPO in CSF,’’ Dr. Jumbe noted. The study is being redone to correct design flaws.
"The presence of EPO and EPO-R in the CNS is well established and the role of EPO in the CNS appears to be neuroprotective," Dr. Jumbe summarized. He added, however, that "there might be a host of other roles." Available data does not support a link in the regulation of CNS and systemic erythropoietic levels. Mechanisms of EPO action and transport across permeability barriers still need to be determined, Dr. Jumbe concluded.