Collaborative Program for Multiple Myeloma
NEW CANAAN, Connecticut-The Multiple Myeloma Research Foundation (MMRF) has announced the recipients of the first MMRF Collaborative Program Grant, which is funding $1.5 million over 3 years to three centers of excellence in myeloma
NEW CANAAN, ConnecticutThe Multiple Myeloma Research Foundation (MMRF) has announced the recipients of the first MMRF Collaborative Program Grant, which is funding $1.5 million over 3 years to three centers of excellence in myeloma research: Dana-Farber Cancer Institute, Mayo Clinic, and H. Lee Moffitt Cancer Center.
The winning grant, entitled "Development of Novel Target-based Therapies for Multiple Myeloma," encompasses three inter-related projects and one unifying core. The focus will be to identify new therapies and rapidly move promising leads from the bench to the bedside for evaluation in clinical trials at all three institutions.
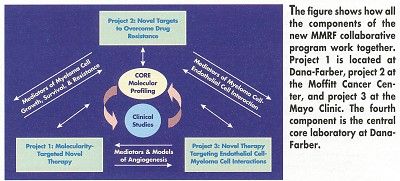
Project 1 will characterize the cell pathways involved in myeloma cell growth, survival, and migration in the bone marrow environment to identify novel molecular targets for therapy. Centered at Dana-Farber, this project is headed by Kenneth C. Anderson, MD, who will also serve as the principal investigator for the entire program. Project 2, centered at the Moffitt Cancer Center and headed by William Dalton, MD, will investigate how myeloma cells become resistant to therapy, with the goal of designing strategies to prevent or overcome drug resistance.
Project 3 at the Mayo Clinic will evaluate the role of angiogenesis in disease progression. It is led by Robert Kyle, MD, Vincent Rajkumar, MD, and Stephen Russell, MD, PhD. The fourth component is the central core laboratory that will perform gene analysis for the entire program. It is headed by Nikhil C. Munshi, MD, of Dana-Farber.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.