FDG-PET Useful in Newly Diagnosed and Recurrent NSCLC
EAST MELBOURNE, Australia-Two studies from the Peter MacCallum Cancer Institute, East Melbourne, Australia, have shown the utility of 18F-FDG-PET for newly diagnosed and suspected recurrent non-small-cell lung cancer (NSCLC). These
EAST MELBOURNE, AustraliaTwo studies from the Peter MacCallum Cancer Institute, East Melbourne, Australia, have shown the utility of 18F-FDG-PET for newly diagnosed and suspected recurrent non-small-cell lung cancer (NSCLC). These studies evaluated the impact of PET in routine clinical practice within a tertiary oncology facility, said Rodney J. Hicks, MD, lead author of both studies.
For 153 consecutive patients with newly diagnosed NSCLC, the researchers compared the treatment plan based on conventional staging methods with the treatment plan based on incorporation of PET findings. The results showed that 10% of cases were downstaged and 33% were upstaged by inclusion of PET.
Among patients with assessable tumors, the PET stage was confirmed in 89%. PET had a high impact on 54 patients (35%), including 34 whose treatment was changed from curative to palliative (see Figure 1), 6 whose therapy was changed from palliative to curative, and 14 whose treatment modality was changed but not the treatment intent. For 39 patients (25%), a previously selected therapy was changed because of the PET results (
J Nucl Med
42:1596-1604, 2001).
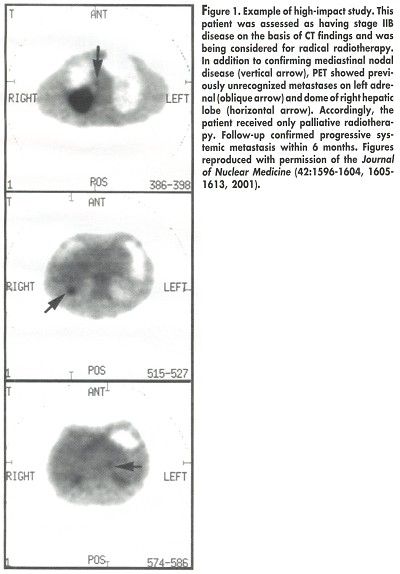
"Staging that incorporated PET provided a more accurate prognostic stratification than did staging based on conventional investigations," Dr. Hicks and his colleagues concluded. "Further, the additional information provided by PET significantly and appropriately changed management in the majority of patients."
The second trial of F-18-FDG PET involved 63 consecutive NSCLC patients with suspected relapse more than 6 months after definitive treatment. The researchers compared the apparent extent of disease on conventional restaging with that on FDG-PET. In order to validate diagnostic findings, serial imaging and pathologic results were obtained during a median follow-up of 19 months.
98% Sensitivity
PET was positive in 41 of 42 patients with confirmed relapse (98% sensitivity). There was no evidence of disease during a minimum follow-up of 12 months in 14 of 15 patients with clinically suspected relapse but negative PET findings (negative predictive value, 93%) (J Nucl Med 42:1605-1613, 2001).
PET resulted in a major management change in 40 patients (63%). In 6 patients, treatment was changed from curative to palliative; in 3 patients, treatment was changed from palliative to curative; and in 9 patients with negative PET findings, treatment was avoided (see Figure 2).
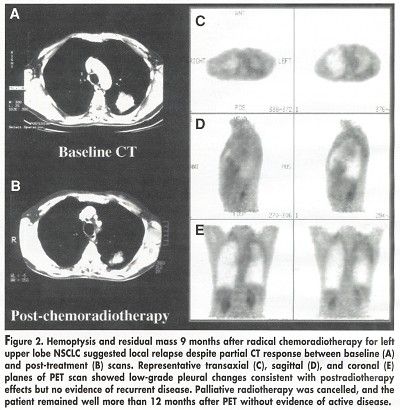
Both the presence of relapse (P = .012) and the extent of relapse (P < .0001) on PET were highly significant prognostic factors.
"PET better assesses the status of disease and stratifies prognosis than does conventional staging, affects patient management, and should be incorporated into paradigms for suspected recurrence of NSCLC," the authors concluded.