Commentary (Vergote): Management of Early Ovarian Cancer
In this issue of ONCOLOGY, Sonodaprovides a systematic reviewof the management of early ovariancancer. The author rightfully concludesthat comprehensive surgicalstaging should be performed in thesepatients and that, based on severalEuropean randomized studies, patientswith high-risk early ovarian cancershould be treated with adjuvant platinum-based chemotherapy. Importantquestions remain, however, including:How should high-risk early ovariancancer be defined? and Is there a needfor adjuvant chemotherapy in patientswho have undergone comprehensivesurgical staging?
In this issue of ONCOLOGY, Sonoda provides a systematic review of the management of early ovarian cancer. The author rightfully concludes that comprehensive surgical staging should be performed in these patients and that, based on several European randomized studies, patients with high-risk early ovarian cancer should be treated with adjuvant platinum- based chemotherapy. Important questions remain, however, including: How should high-risk early ovarian cancer be defined? and Is there a need for adjuvant chemotherapy in patients who have undergone comprehensive surgical staging? Reclassifying Early Ovarian Cancer
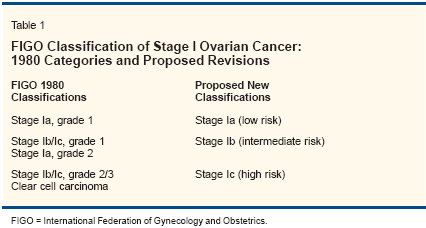
The term "early ovarian cancer" is used to describe disease confined to one or both ovaries (International Federation of Gynecology and Obstetrics [FIGO] stage I) or limited in spread to the true pelvis (FIGO stage II). Because most patients with FIGO stage II disease are surgically and medically treated in the same manner as advanced ovarian cancer patients, we will focus here on stage I disease. The largest study[1] of prognostic factors in stage I disease (N = 1,545) confirmed earlier studies, that degree of differentiation is the most important independent prognostic factor in stage I disease. Hence, it seems indicated to include the degree of differentiation in the FIGO classification and clinical decision-making. Furthermore, it has been shown that FIGO stage Ic disease has a similar prognosis as stage Ib.[1,2] It is, therefore, time for a new FIGO classification that takes into account these findings. Table 1 offers a revised classification for low (Ia), intermediate (Ib), and high-risk (Ic) early ovarian cancer. In the future, newer prognostic factors such as DNA ploidy or other biologic markers analyzed with immunohistochemistry or newer techniques such as microarrays or proteomic analysis will probably replace the clinical prognostic variables used in this classification.
ACTION and ICON1 Trials
The combined analysis of the European Organization for Research and Treatment of Cancer's Adjuvant Chemotherapy in Ovarian Neoplasms (ACTION) trial and the International Collaborative Ovarian Neoplasm (ICON1) trial showed for the first time that adjuvant platinum-based chemotherapy improves overall survival in early ovarian cancer compared with observation.[3] Because other regimens, including taxanes, were not studied in comparison with a no-treatment arm, it is probably appropriate to extrapolate from the experience in advanced ovarian carcinoma that the addition of paclitaxel to carboplatin (Paraplatin) is warranted in high-risk stage I ovarian carcinoma. Comprehensive surgical staging (including blind biopsies of, for example, diaphragm and paracolic gutters, omentectomy, and para-aortic and pelvic lymphadenectomy) has resulted in upstaging of about one-third of the patients believed to have stage I disease. In a subgroup analysis of the ACTION trial, no difference in overall survival was observed between the observation and adjuvant platin chemotherapy groups. However, final conclusions cannot be drawn from this subgroup analysis of the ACTION trial due to the low number of events. Ideally, a new randomized study that compares restaging with adjuvant chemotherapy in nonoptimally surgically staged patients should be performed. However, such a study does not seem feasible given the low number of recurrences among optimally surgically staged patients, the low incidence of stage I ovarian carcinoma, and the fact that combined analysis of the ICON1 and ACTION trials showed a benefit for adjuvant platinum-based chemotherapy. Hence, comprehensive surgical staging and adjuvant platinum- based chemotherapy is currently indicated for all patients with high- or intermediate-risk stage I invasive epithelial ovarian carcinoma who are fit enough to undergo such treatment.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Vergote I, De Brabanter J, Fyles A, et al: Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 357:176-182, 2001.
2. Trimbos JB, Vergote I, Bolis G, et al: Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian cancer: European Organization for Research and Treatment of Cancer–Adjuvant Chemotherapy in Ovarian Neoplasms Trial. J Natl Cancer Inst 95:113- 125, 2003.
3. Trimbos JB, Parmar M, Vergote I, et al: International Collaborative Ovarian Neoplasm and International Collaborative Ovarian Neoplasm: Two parallel phase III trials of adjuvant chemotherapy in patients with early-stage ovarian cancer. J Natl Cancer Inst 95:105-112, 2003.