Computer Aided Detection Helps Radiologists Find Lung Nodules
This special “annual highlights” supplement to Oncology News International is acompilation of major advances in the management of lung cancer during 2004, asreported in ONI. Guest editor Dr. Roy Herbst discusses these advances in clinicalmanagement, with a focus on developments in adjuvant therapy for early disease,targeted therapy, and new chemotherapy findings.
SUNNYVALE, California-R2Technology, Inc., has received approvalfrom the US Food and DrugAdministration (FDA) for itsImageChecker CT Computer AidedDetection (CAD) Software system toassist radiologists in the detection ofsolid pulmonary nodules (between 4mm and 30 mm in diameter) duringreview of multidetector CT scans ofthe chest. It is intended to be used asan adjunct, alerting the radiologist-after his or her initial reading of thescan-to regions of interest that mayhave been initially overlooked.
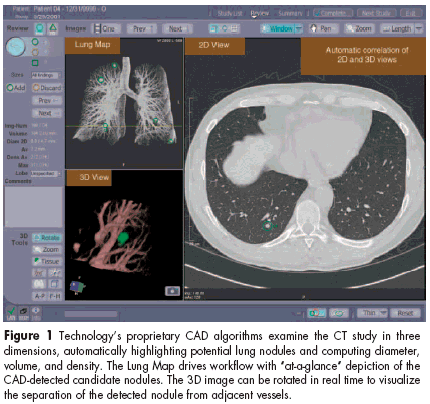
The ImageChecker CAD algorithms examine the complete set of CT imagesgenerated during scanning andsearch for findings with features suggestiveof a solid lung nodule. Thesystem marks these candidate nodulesfor further review by the radiologist.The system workstation is designed to improve radiologist efficiencyin reviewing chest CT examsthrough work-flow-enhancing toolsand through automatic measurementand characterization of the detectedlung nodules.FDA approved the system basedon a clinical study showing that useof the ImageChecker CT CAD system significantly (P = .003) increasedthe detection of actionable (requiringintervention or short-term follow-up)solid lung nodules by calling attentionto potential abnormalities. In thestudy, 15 radiologists independentlyreviewed 90 cases from lung CT scansfirst without the ImageChecker CTsystem and then again with the system.The system had a false markerrate of only two false marks per case,measured on cases with an average ofmore than 170 slices per case.The labeling for the ImageCheckerCT CAD system includes instructionsthat the radiologist should always readthe film before using the CAD systemand never change an original positivereading to a negative one becauseCAD did not identify the nodule.R2 Technology also announcedFDA clearance to market two softwarepackages for use with theImageChecker CT system. One coversthe Temporal Comparison softwaremodule, which provides spontaneousthree-dimensional registrationand the ability to automaticallytrack lung nodule progressionor regression over time. Growth rateis an important indicator of clinicalsignificance, and automating this processhas potential for significant timesavings, the company said.The second clearance is for the FillingDefect Indicator software module,designed to help physicians visualizeand evaluate filling defects inpulmonary arteries, such as pulmonaryemboli.