UFT Provides ‘Equivalent’ Survival and Quality of Life to 5-FU in Stage II/III Colorectal Cancer
The 30 reports in this special supplement to Oncology News International represent highlights of ongoing major clinical trials and new research presented at ASCO 2004 regarding state-of-the-art chemotherapeutic management of gastrointestinal and other cancers. Important developments in capecitabine as adjuvant therapy, novel targeted agents, and new combinations are discussed.
PITTSBURGH-Compared tofluorouracil (5-FU), oral tegafur/uracil (UFT) provided "equivalent"survival, similar toxicity, and significantquality-of-life improvements ina large clinical trial among patientswith stage II/III colorectal cancer.Norman Wolmark, MD, chair ofthe National Surgical Adjuvant Breastand Bowel Project (NSABP), reportedthe results of NSABP Protocol C-06, which compared 5-FU/leucovorinto UFT/leucovorin in more than1,600 patients (abstract 3508). Previousrandomized trials have suggestedthat UFT offers efficacy and safetysimilar to what is obtained with standardfirst-line therapy for metastaticcolorectal cancer.Interest Peaked in 1990s
Interest in the use of UFT for colorectalcancer peaked in the 1990s,after presentation and publication oftwo randomized trials that examinedthis agent disease as a first-line treatmentfor metastatic disease. Both comparedsimilar doses of UFT/leucovorinwith the approved Mayo Clinic5-FU/leucovorin regimen. Nearly1,200 patients were enrolled in thosetrials (J Clin Oncol 20(11):3605-3616,2002; J Clin Oncol 20(17):3617-3627,2002).
The hazard ratio for survival in thefirst trial favored 5-FU (HR = 0.96),while the hazard ratio in the secondtrial favored UFT (HR = 1.14). Onecan conclude there is very little differencein survival between the two arms,"Dr. Wolmark noted.Those data were presented to theOncology Drug Advisory Committee(ODAC), which made a unanimousrecommendation that the drug be ap-proved. However, "FDA declined toaccept the recommendation," Dr.Wolmark said.Thus, it was "with a certain degreeof dysphoria,'' said Dr. Wolmark, thathe presented these results, "knowingthat UFT is available for use in justabout every country in the world, withthe exception of the United States."Exceeds Efficacy Threshold
In Protocol C-06, Dr. Wolmark andcolleagues randomized patients withstage II/III colorectal cancer to receiveeither:
- the Roswell Park regimen of5-FU/leucovorin, consisting of 5-FU500 mg/m2 IV bolus and leucovorin500 mg/m2 IV weekly for 6 weeks,followed by a 2-week rest period; thiswas repeated for three cycles; or
- UFT 300 mg/m2/d PO and leucovorin90 mg/d PO for 28 days, followedby a 1-week rest period; this wasrepeated for five cycles.
Over a 2-year period ending March1999, a total of 1,608 patients wereenrolled. The primary aim of the studywas to "compare the relative efficacy"of the regimens in prolonging survival,according to Dr. Wolmark. Thesecondary objective focused on qualityof life issues.Five-year survival was 78.7% inboth the UFT and 5-FU arms. By prespecifiedcriteria, investigators determinedthat they would need a P valuegreater than .39 to consider the treatmentsequivalent; in fact, the P valuewas .88, which "certainly" exceeds theprespecified threshold, Dr. Wolmarknoted.In addition, 5-year relapse-free survivalwas not significantly differentbetween arms, at 76.4% for 5-FU/leucovorinand 74.5% for UFT/leucovorin(
P
= .62). Similarly, disease-freesurvival at 5 years was 68.3% for 5-FU/leucovorin and 66.9% for UFT/leucovorin (
P
= .79), with "virtuallysuperimposable" survival curves, Dr.Wolmark said.
Quality of Life Issues
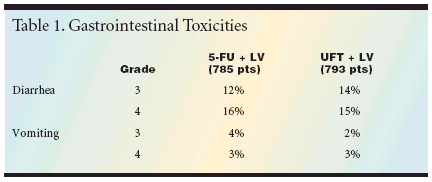
Toxicities in the NSABP study weremainly gastrointestinal with both regimens.There was a "very similar" distributionof grade 3/4 adverse events,including diarrhea, vomiting, and nausea,Dr. Wolmark reported. The incidencesin both treatment arms were18% for grade 3 events and 19% forgrade 4 events (see Table 1).Quality-of-life outcomes as measuredby the SF-36 vitality scale, thesymptom distress scale, symptomchecklist, and burden of care questionsfavored the UFT arm. There wasno difference in several other parameters,including the colon-specificFACT-C score, return to normal activity,and overall quality of life."For those in the audience whotreat patients in the United States, youneed not encumber yourself with thesedata, or with the nuances of this presentation,"Dr. Wolmark said ina somewhat tongue-in-cheek commentaryon the results, "becauseUFT is not approved in the UnitedStates."