Concurrent Rituximab and Fludarabine Has a Higher Complete Response Rate Than Sequential Treatment in Untreated Chronic Lymphocytic Leukemia Patients: Results From CALGB 9712
Rituximab (Rituxan), a chimeric anti-CD20 antibody, is effective as a single agent in chronic lymphocytic leukemia (CLL), down-regulates antiapoptotic proteins in CLL cells in vivo, and is minimally immunosuppressive.
Rituximab (Rituxan), a chimeric anti-CD20 antibody, is effective as a singleagent in chronic lymphocytic leukemia (CLL), down-regulates antiapoptoticproteins in CLL cells in vivo, and is minimally immunosuppressive. To furtherimprove outcomes of previously untreated CLL patients receiving fludarabine(Fludara), the Cancer and Leukemia Group B (CALGB) conducted a randomized phaseII trial of sequential vs concurrent rituximab.
All patients fulfilled the National Cancer Institute (NCI) definition of CLLand were symptomatic. Patients on the sequential arm (fludarabine followed byrituximab) received fludarabine (25 mg/m² days 1-5 every month × 6) inductionfollowed 2 months later by rituximab (375 mg/m² repeated weekly × 4). Patientson the concurrent arm (fludarabine with rituximab) received therapy identical tothe sequential arm, except rituximab (375 mg/m²) was also administered (days 1and 4 of cycle 1; day 1 of cycles 2-6) with induction therapy. A total of 104patients were enrolled. Median age was 64 years (range: 36-86 years). ModifiedRai stage class was 59% intermediate and 41% high risk. Fludarabine inductiontoxicity included neutropenia (grade 3, 11%; grade 4, 30%), thrombocytopenia(grade 3, 8%; grade 4, 4%), and infection (grade 3, 19%; grade 4, 2%).
Nine of the first 44 patients (20%) on the concurrent fludarabine andrituximab induction arm developed grade 3/4 dyspnea or hypotension withfull-dose rituximab on day 1 of therapy, as compared to none of the last sevenpatients utilizing stepped-up dosing (50 mg/m² day 1, 325 mg/m² day 3, 375mg/m²day 5 of cycle 1 only). Other toxicities included neutropenia (grade 3, 31%;grade 4, 43%), thrombocytopenia (grade 3, 14%; grade 4, 6%), and infection(grade 3, 18%). Consolidation was tolerated well in both arms; grade 3/4neutropenia was more common in the concurrent arm vs the sequential arm (37% vs 18%). Opportunistic infections were noted in 8 patients on the concurrent armand 14 patients on the sequential arm. Fifteen of these were varicella-zoster (n= 5) or herpes simplex 1/2 (n = 10).
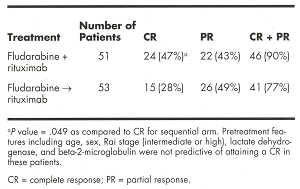
Response data using the modified NCI criteria are summarized in the tableabove.
CONCLUSION: These data suggest that the concurrent combination of rituximabwith fludarabine is feasible after management of initial infusion toxicity forwhich a stepped-up dosing schedule has been introduced. Overall, toxicity on thetwo arms was similar. Complete response was more frequent on the concurrent armthan on the sequential arm, and toxicities were similar and acceptable. We planto test concurrent fludarabine and rituximab in a phase III trial.