Rituximab Treatment in Patients With Immune Thrombocytopenic Purpura
Immune thrombocytopenic purpura (ITP) in adults is a chronic autoimmune disease characterized by antiplatelet antibody (APA)-mediated thrombocytopenia.
Immune thrombocytopenic purpura (ITP) in adults is a chronic autoimmunedisease characterized by antiplatelet antibody (APA)-mediated thrombocytopenia.The aim of treatment is to maintain a safe platelet count with minimal toxicity.Rituximab (Rituxan) is a chimeric anti-CD20 monoclonal antibody that depletes Blymphocytes and is used to treat patients with B-cell lymphomas. There is littletoxicity apart from first infusion reactions, which are rarely severe.
As ITP is an antibody-mediated disease, this trial aimed to assess theefficacy of rituximab in adults with primary ITP refractory to multipletreatments with platelet counts below 30,000/µL. Patients received 375 mg/m² ofrituximab weekly for 4 weeks. Response was defined as a platelet increase tomore than 50,000/µL. A total of 21 patients have enrolled, 14 of which have beenfollowed for more than 10 weeks and are summarized in the table below:
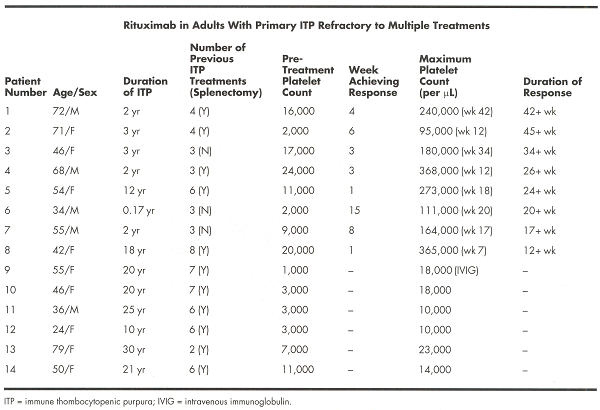
Of 14 patients, 8 (57%) responded a median of 3.5 weeks (range: 1-15 weeks)from first infusion; all are still maintaining platelets above 50,000/µL at amedian of 25 weeks follow-up. Of those eight, five achieved complete remission(CR) at a median of 14 weeks. Males, patients diagnosed with ITP for < 3years, and those with higher initial platelet counts were more likely torespond. Only one patient (number 13 in the table) had detectable CD19-positivelymphocytes by week 12 of treatment.
There were no serious infections and no significant change in immunoglobulin(Ig) levels. Of 21 patients, 8 experienced transitory first infusion reactionssuch as red face, hives, itching, and throat discomfort. Two patients have aT-cell clone (one responder and one nonresponder) and one a B-cell clone(nonresponder). The clones did not disappear after treatment. IgM and IgG APAlevels are being assessed.
CONCLUSION: In summary, 57% of adults with difficult ITP have responded totreatment, with 36% in CR. Rituximab is especially useful because of its longduration of response in both patients with recently diagnosed ITP, allowingresponders to postpone splenectomy, and also in patients refractory to standardtherapies. The mechanism of action of rituximab in ITP is unclear. The timing ofresponse makes it unlikely that response is only related to decreased levels ofAPA, as antibody-producing plasma cells, especially IgG-producing, infrequentlyexpress CD20.