Incidental identification of small renal masses (SRMs) has become increasingly common with widespread adoption of cross-sectional imaging. To date, early detection of SRMs has not translated to a substantial improvement in cancer-specific survival. Guidelines on the management of SRMs are evolving to reflect recent developments in treatment. The major approaches to managing SRMs include active surveillance, partial/radical nephrectomy, and ablative therapies, such as radiofrequency ablation with cryoablation. The goal of treatment is to optimize oncologic and renal function outcomes while avoiding overtreatment and associated morbidity. In this review, we summarize the diagnosis of SRMs, the role of renal mass biopsy, different treatment strategies, and future directions, including emerging molecular biomarkers.
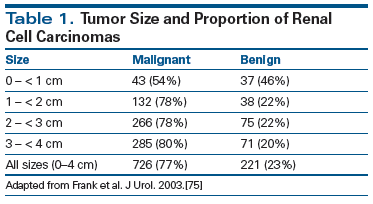
Evaluation and management of small renal masses (SRMs)- defined as enhancing renal masses < 4 cm on axial imaging-have become important clinical issues in recent years, particularly in light of the increase in incidentally detected renal lesions that has followed marked growth in the use of abdominal imaging, such as computed tomography (CT)/magnetic resonance imaging (MRI) and ultrasound.[1-3] Use of CT scanning has become widespread, with recent studies showing scans increasing by 330% over the last decade in some clinical settings.[4] Recent estimates suggest that CT scans detect incidental renal lesions measuring at least 1 cm or larger in 14% of asymptomatic adults who undergo imaging for other indications.[5] Although the likelihood of malignancy increases with mass size, more than 20% of incidentally detected renal masses are benign, and current imaging modalities cannot reliably distinguish malignant tumors from benign tumors, such as oncocytomas and lipid-poor angiomyolipomas (Table 1).[6]
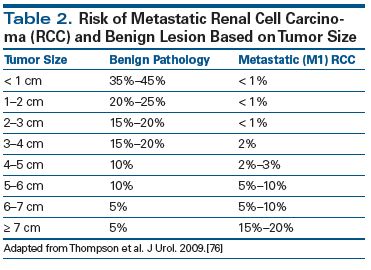
The incidence of renal cell carcinoma (RCC) has increased in parallel with the burgeoning detection of SRMs. In fact, SRMs are the predominant driving factor in the upward trend in kidney cancer incidence noted over the last 20 years. An estimated 60,000 new kidney cancers were diagnosed in 2015 in the United States, 40% of which were less than 7 cm at detection (stage I size cut point).[7] As a result, the historical triad of flank pain, hematuria, and flank mass is now rare, accounting for less than 10% of RCC cases.[8] However, despite this downward stage migration, kidney cancer–specific mortality has not decreased significantly, and 30% of RCC patients present with metastatic disease at diagnosis.[9] Among SRMs, the likelihood of metastatic disease is highly dependent on tumor size. For example, Lee and colleagues found an incremental increase in metastases across tumor size groups (1–2 cm, 1.1%; 2–3 cm, 3.3%; 3–4 cm, 6%) in patients with clinical T1a SRMs (Table 2).[10] The economic burden of kidney cancer is considerable. Medicare expenditures alone surpass $26,000 per year per patient,[11,12] which includes evaluation and management of SRMs.
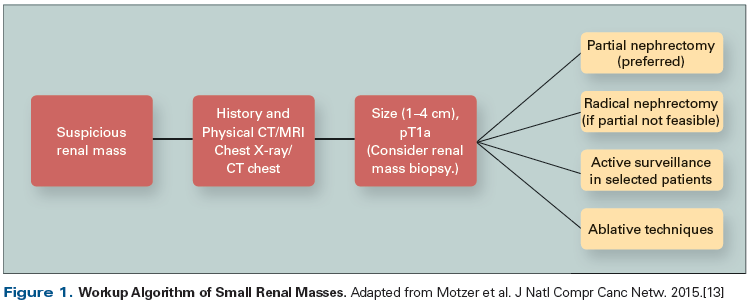
Given the prevalence and growing clinical importance of SRMs, evaluation and management recommendations have been released by a number of groups. For example, current American Urological Association (AUA), European Association of Urology (EAU), and National Comprehensive Cancer Network (NCCN) recommendations provide guidance regarding management strategies that include active surveillance, surgical extirpation, and ablation (Figure 1).[13] Although several patient factors, such as age, medical comorbidities, and patient preferences affect the decision-making process, optimal management of SRMs should balance the need to treat/remove a potential cancer, with the salutary objective of preserving as much renal function as possible.[1] In this review, we provide an overview of current diagnostic and evaluative issues surrounding the management of SRMs, review the role of renal mass biopsy, and discuss management options including active surveillance, surgery (partial nephrectomy and radical nephrectomy), and ablation therapy.
Recent Advances in Imaging Evaluation
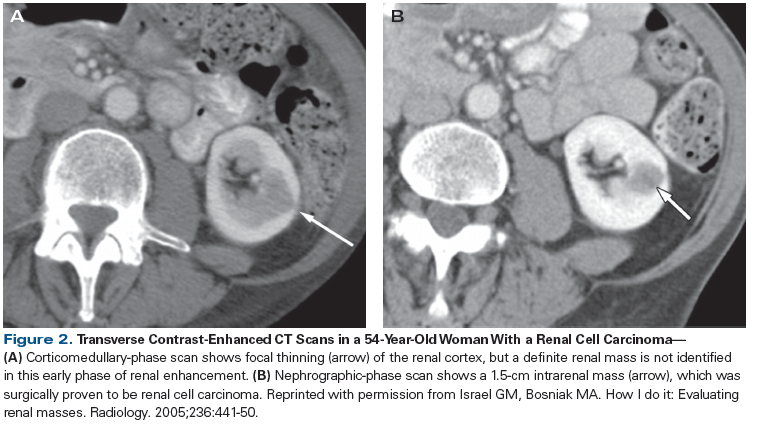
The gold standard of SRM imaging is axial imaging in the form of CT scan or MRI.[1] Intravenous contrast administration is essential to confirm enhancement of the renal mass. Enhancement is defined as an increase in Hounsfield unit measurement of greater than 20 on post-contrast images (Figure 2).[14] MRI is equivalent to CT in determining enhancing SRMs and may be able to detect histologic subtypes of RCC.[15] However, CT and MRI are unable to distinguish benign oncocytoma from RCC.[1] Recent imaging advances also represent the important area of risk stratification of SRMs. Gorin et al reported on a novel application of an imaging technique in SRMs to distinguish benign oncocytic tumors from mixed malignant chromophobe tumors. Utilizing 99mTc-sestamibi single-photon emission computed tomography in 50 patients with SRMs, blinded radiologists successfully identified 5 of 6 oncocytomas and 2 of 2 hybrid oncocytic/chromophobe tumors.[16] In addition, there has been research in G250 (an antigen to carbonic anhydrase IX present on clear cell RCC) PET/CT using iodine-124–labeled imaging to distinguish between malignant and benign lesions.[17] In a preclinical mouse model trial, investigators demonstrated efficacy of using dual-labeled anti–carbonic anhydrase IX antibody G250 to identify clear cell RCC.[18] The major limitations of these specialized imaging techniques are financial, and further research is needed to identify a cost-effective method to risk-stratify SRMs based on imaging characteristics.
Renal Mass Biopsy
There has been some expansion of the role of percutaneous renal mass biopsy in evaluating SRMs.[19] The major question posited prior to performing biopsy relates to meaningful and actionable information gained. (That is, will the results change patient management?) In a meta-analysis of 57 studies, core needle biopsies had superior accuracy compared with fine needle aspiration.[1] Recent articles, including one by Richard et al, advocate widespread implementation of renal mass biopsy in every case of SRMs because of the high diagnostic yield (accuracy > 90%) and low associated morbidity (major bleeding < 1%).[1,20] Also, the theoretical risk of tumor cell seeding on the biopsy track is extremely rare, with only a handful of case reports in the literature.[21] In patients with an indeterminate biopsy, repeat biopsy enabled the physician to make a definitive diagnosis in 80% of cases at one academic center.[22]
Retrospective studies have suggested that up to half of partial nephrectomies could have been avoided by preoperative renal mass biopsy (assuming 100% perfect concordance with a paradigm of surveillance for low-risk cancers and not just benign tumors).[23] Renal mass biopsy has gained momentum as patients with indeterminate oncocytic neoplasms have been safely followed with active surveillance.[24] EAU and AUA guidelines recommend renal mass biopsy in cases prior to ablation or when active surveillance is pursued.[1,7]
Despite this momentum, renal mass biopsy fails to accurately identify high-grade tumors, given the intermixing of high- and low-grade elements in the majority of tumors, resulting in approximately 62% accuracy.[1] Recently, Su et al presented the first clinical study of real-time fiber-optic confocal laser endomicroscopy for SRMs.[25] The study was performed on ex vivo specimens, but being able to identify malignant and possibly more aggressive portions of the tumor would allow for higher yield on targeted biopsies moving forward. Other active research currently underway at the University of Michigan is examining molecular markers on renal mass biopsy tissue, raising the possibility that individualized molecular markers derived from biopsy tissue will be able to better assess risk of cancer-related mortality and recurrence in the future.[26]
Active Surveillance
Over the past decade, there has been a major focus on preservation of renal function and prevention of overtreatment of relatively innocuous malignancies. For this reason, active surveillance, defined as serial radiographic imaging to determine growth kinetics of SRMs, is an attractive option. Studies investigating linear growth rate of SRMs demonstrate an average linear growth rate of < 0.5 cm/year.[27] AUA guidelines recommend that active surveillance be included in the discussion with every patient. In the elderly or patients with comorbidities, active surveillance should be considered acceptable in the management of SRMs to delay or avoid treatment in higher-risk patients.[7] EAU guidelines recommend active surveillance or ablation in elderly or comorbid patients, but because of the paucity of evidence, do not specify a preference.[1] There are no universally accepted protocols for repeat imaging; however, most centers reimage at 6-month intervals. The decision to pursue definitive treatment is based largely on linear growth rate, especially > 0.5 cm/year.[28] In a large active surveillance series, factors associated with disease progression were larger tumor size at presentation, advanced age, and elevated linear growth rate.[29]
Analysis of the largest nonrandomized prospective observation cohort, the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry, demonstrated that patients on active surveillance who had renal functional decline had worse renal function than what would be expected from aging alone, likely reflecting increased chronic medical comorbidities, including hypertension, diabetes, and potential replacement of functioning parenchyma by tumor. Patients who chose active surveillance were older, had poorer performance status, and smaller tumors. Five-year overall survival was worse in the active surveillance group (75% vs 92% at 5 years), but cancer-specific survival was equivalent.[28] Regarding cost-effectiveness of active surveillance vs primary treatment (ablation), one retrospective study demonstrated that active surveillance with delayed cryoablation was similar in cost-effectiveness and oncologic outcomes compared with upfront cryoablation.[30] Another study suggested that renal mass biopsy may not only prevent unnecessary surgery by effectively directing patients to active surveillance, but may also be part of a cost-effective paradigm for management of SRMs.[31]
Partial and Radical Nephrectomy
The current standard of surgical management of SRMs is nephron-sparing surgery (eg, partial nephrectomy), which was first reported by Czerny in 1890.[32] Guidelines recommend partial nephrectomy as the preferred surgical management in patients with a clinical T1 renal mass.[1,7] Historically, partial nephrectomy was reserved for select patients with renal masses, including patients with solitary kidney, pre-existing chronic kidney disease (CKD), or bilateral renal tumors.[33] However, the preponderance of evidence suggests that partial nephrectomy is equivalent in terms of oncologic outcomes and superior in quality-of-life measurements, including regret over loss of the entire kidney and long-term renal function.[34-36] A long-term comparison of patients treated with partial nephrectomy vs total/radical nephrectomy for tumors ≤ 5 cm reported equivalent cancer-specific survival and local recurrence rates at 9 years of follow-up.[37] Perioperative morbidity is slightly higher following partial nephrectomy compared with complete kidney removal for several complications, including bleeding (3.1% vs 1.2%), urine leak (4.4% vs 0%), and need for reoperation (4.4% vs 2.4%).[38] A prospective randomized trial comparing partial nephrectomy with total nephrectomy in 541 patients with small (< 5 cm) renal masses unexpectedly found higher overall survival in the total nephrectomy group (81% vs 76%, respectively; P < .05) at a mean follow-up of 9.3 years.[39] However, cardiovascular deaths were more common in patients who underwent partial nephrectomy, despite lower burdens of CKD. This trial (European Organisation for Research and Treatment of Cancer [EORTC] 30904) has been criticized for several shortcomings, including marginally statistically significant results and inclusion criteria that were not limited to kidney cancers. Overall survival was equivalent when the trial data were analyzed excluding patients with benign pathology.[39]
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"49613","attributes":{"alt":"","class":"media-image","id":"media_crop_993831172999","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"6010","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","style":"height: 162px; width: 144px;","title":" ","typeof":"foaf:Image"}}]]
Robert Uzzo, MD
Miki Haifler, MD
Fox Chase Cancer Center,
Temple Health
Philadelphia, PennsylvaniaWhat Are the Key Issues Surrounding Small Renal Masses (SRMs)?The incidental finding of a renal mass has become more common in the last several decades, coinciding with improvements in axial imaging. Approximately 20% of these tumors are benign. However, modern imaging modalities are unable to differentiate reliably between benign and malignant tumors. Even renal biopsy is not sufficiently accurate in identifying high-grade malignant tumors. Therefore, the preferred treatment nowadays is surgical resection, mainly by partial nephrectomy. In select patients with normal contralateral kidney and competing risks, radical nephrectomy is also a viable option.How Does This Translate Into a Treatment Dilemma? In order to decrease the morbidity of extirpative surgery, less invasive treatments are offered, including thermal ablation and active surveillance. The American Urological Association and European Association of Urology guidelines recommend these options for high-risk surgical candidates, such as the elderly and patients with comorbidities. These patients have competing risks in addition to the renal tumor, and may benefit from renal mass biopsy most, since it enables the urologist to better risk-stratify them into high- and low-risk tumor groups, and thus offer the optimal personalized treatment option.What Are the Future Directions for SRMs? Ongoing research into subdividing SRMs into high- and low-risk tumors include genomic testing (eg, microsatellite instability) and urine biomarkers (eg, aquaporin 1). These tests will enable the differentiation of benign and malignant tumors and will distinguish aggressive and indolent subtypes of renal cancer. Furthermore, research into new imaging modalities (eg, single-photon emission computed tomography) may enable the radiologist to provide information on the biologic nature of SRMs.
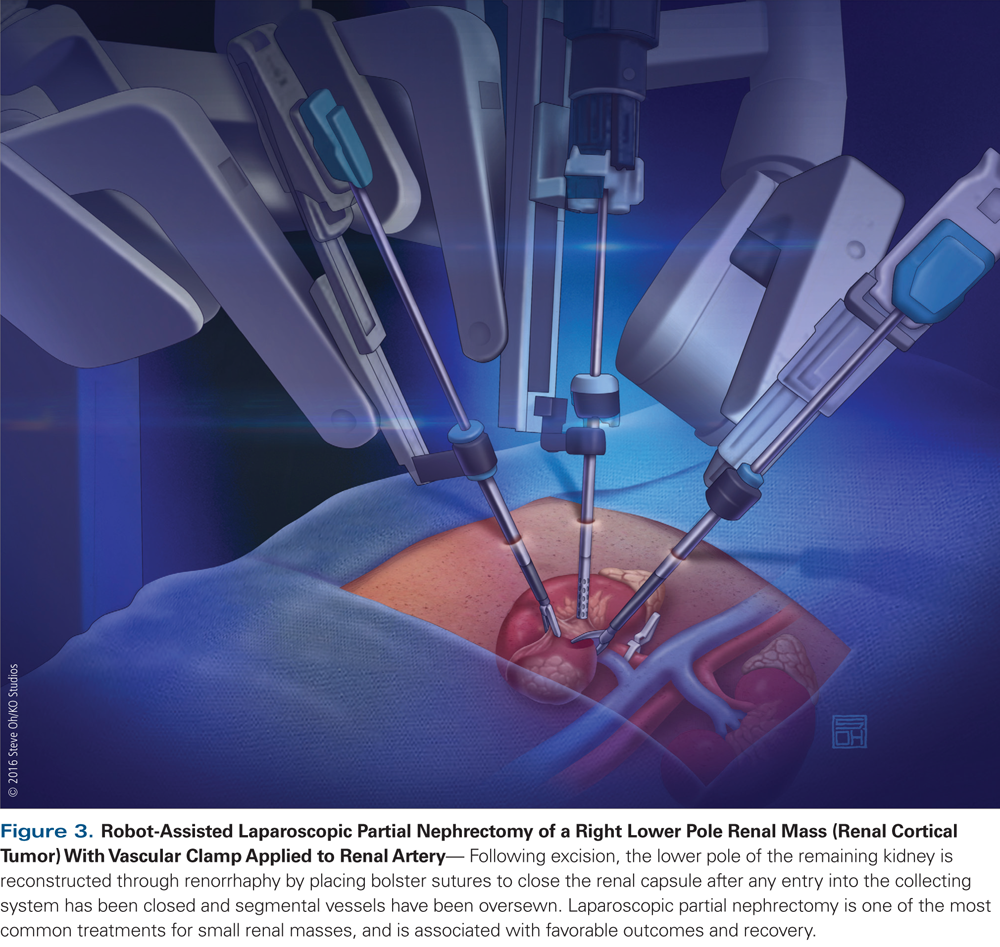
Minimally invasive surgical (MIS) approaches to partial nephrectomy and radical nephrectomy, including laparoscopic and robotic-assisted approaches, are increasingly utilized.[40] With regard to open vs MIS partial nephrectomy, oncologic outcomes and renal function outcomes were found to be equivalent, with lower blood loss but longer operative times observed in laparoscopic approaches (Figure 3).[41-43] Nephrometry scores (Radius, Exophytic/Endophytic, Nearness to collecting system or sinus, Anterior/posterior, and Location relative to polar lines [RENAL]; Preoperative Aspects and Dimensions Used for Anatomic classification [PADUA]; centrality index) based upon preoperative imaging characteristics of SRMs have been widely implemented to objectively categorize operative complexity of partial nephrectomy.[44-46] The RENAL nephrometry score is the most widely used and has allowed for standardization in reporting outcomes and complications after partial nephrectomy. In addition, use of RENAL nephrometry is correlated to perioperative renal impairment.[47] Recently, Spaliviero et al have proposed a new nephrometry method called arterial-based complexity. This scoring system is based upon size of renal arterial branches pertinent to the tumor dissection. Further research is needed to validate this scoring system in a multi-institutional model.[48]
The goal of nephron-sparing surgery is to optimize renal function postoperatively while not compromising oncologic outcomes. The most important determinants of postoperative renal function are preoperative renal function, volume of renal parenchyma preserved, and vascular damage to nonmalignant parenchyma. The first factor relates to underlying medical renal disease and is not modifiable, while the volume of remaining parenchyma is largely determined by nonmutable tumor characteristics.[38] Therefore, much emphasis has been placed upon minimizing ischemic injury to nonmalignant tissue by decreasing clamp time or performing off-clamp or selective clamping techniques. Numerous studies have analyzed the optimal method of ischemia to preserve renal functional outcomes.[49] Minimizing ischemia time is accepted as the key factor, and many studies suggest longer ischemia time (> 30 minutes) results in poorer renal function within the first 3 to 6 months postoperatively. However, Castaneda et al retrospectively compared renal functional outcomes at 6 months and 1 year postoperatively and found no difference in main renal artery clamping vs selective clamping vs off-clamp methods.[50] The need to preserve renal function was demonstrated in a population-based study of more than 1 million patients, showing elevated incidence of cardiovascular events and mortality with worsening stages of CKD.[51] The optimal multi-parameter outcome of nephron-sparing surgery has been defined as negative surgical margin, absence of perioperative complications, and warm ischemia time of 25 minutes or less.[52]
Ablation
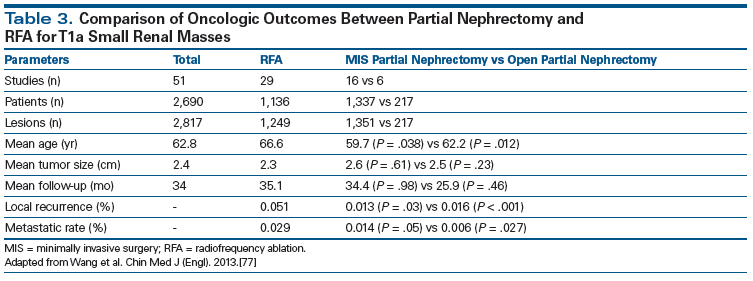
Thermal ablation therapies, including cryoablation and radiofrequency ablation, have emerged as potential treatment options in select elderly or comorbid pateints.[53] AUA and EAU guidelines recommend considering ablative approaches in patients who are at risk of increased surgical morbidity.[1,7] It should be noted, however, that there are insufficient data on long-term oncologic outcomes to recommend ablative therapies as a preferred modality.[1] Approved ablative techniques, including radiofrequency ablation and cryoablation via percutaneous or laparoscopic approaches, show no differences in complication rates or survival outcomes between percutaneous or laparoscopic methods; however, well-designed matched trials are lacking.[54-59] Retrospective studies suggest that partial nephrectomy confers an improved cancer-specific survival compared with ablation.[60] Local recurrence is also more likely following ablation compared with partial nephrectomy, with recurrence rates ranging from 10% to 20%.[7] Renal functional outcomes are comparable to partial nephrectomy, as shown in Table 3.[61] Experimental research is still being conducted with alternative ablative methods such as microwave and high-intensity focused ultrasound and radiosurgery.[62-64] Another recent technology, irreversible electroporation, has been described in small series and may have benefits, given its nonthermal nature.[65] Very small experimental trials exist with these aforementioned modalities, and research is being conducted to determine efficacy and safety.
Renal Preservation and CKD-S vs CKD-M
Recently, there has been a major focus on renal function preservation. However, many questions remain about how to optimize renal function within the context of managing SRMs. For instance, active surveillance is an attractive option because it preserves all renal tissue. However, analysis of the DISSRM registry demonstrated a confounded observation that patients on active surveillance who experience renal functional decline have worse renal function than what would be expected from aging alone.[50] Also, outcomes of patients with CKD from medical disease (CKD-M) was used to justify nephron-sparing surgery early on. However, recent studies have shed light on the distinction of CKD-M compared with CKD due to surgical reasons (CKD-S). Lane et al demonstrated worse outcomes for CKD-M patients vs CKD-S patients.[66] Patients with CKD-S demonstrated a yearly functional decline of 0.7% compared with CKD-M patients, with a renal functional decline of 4.7%.[67] In an updated analysis, Lane et al demonstrated that a baseline glomerular filtration rate (GFR) of greater than 45 mL/min/1.73 m2 (from medical/surgical) predicted worse survival and more rapid functional decline. This suggests that while there may be a difference between CKD-M and CKD-S, patients with anticipated GFR decline to less than 45 mL/min/1.73 m2 postoperatively would benefit most from nephron-sparing approaches.[66] Optimal management of individual patients is still uncertain. In an increasingly cost-conscious age of healthcare, one study suggested that radiofrequency ablation compared with partial nephrectomy may be more cost-effective at 6 months.[68] Another study suggests that active surveillance is the most cost-effective management strategy, followed by ablation and partial nephrectomy.[69]
Future Directions
Emerging areas that may redefine the evaluation and management of SRMs will likely depend upon personalized and precision-based approaches, such as molecular tumor profiling. Two clinical trials in early phases of development, NCT02204800 and NCT01305330, are working to investigate microsatellite instability and chromatin remodeling gene alterations. Urinary biomarkers including aquaporin 1 and perilipin 2 have shown some promise in early clinical trials and have been highly sensitive and specific in distinguishing benign tumors from malignant RCC. Currently, the major limitation of these biomarkers is their inability to detect chromophobe RCC and other subtypes.[70] Simple hematologic tests, including platelet-to-lymphocyte ratio, have been used to predict malignancy in patients with SRMs[71]; however, results have been equivocal. Recent research using genome analysis has been able to stratify cancer aggressiveness based on genomic profiling.[72] In addition, the Cancer Genome Atlas Research Network has identified 19 mutated genes in clear cell RCC, and several genes in papillary RCC correlated to aggressiveness. Decreased expression of a new class of small noncoding RNAs called PIWI-interacting RNAs has also been linked to poorer survival in RCC patients.[73] Ultimately, the translation of such research into practice may aid in distinguishing between patients who require treatment for SRMs and patients who do not require active therapy, obviating the need for expensive treatment, imaging, and surveillance protocols. Additional research is needed to develop a molecular prognostic test for SRMs that stratifies patients with high fidelity, similar to those available for evaluating other cancers.[74]
Conclusions
The evaluation and management of SRMs have evolved over the past few decades. However, despite technological and surgical advances, little survival benefit has been observed. Future trials utilizing precision molecular profiling to risk-stratify patients’ SRMs are needed, and may be the way forward to differentiate aggressive cancers from indolent masses. Without improved knowledge and predictive nomograms derived from such future research, patients and clinicians will continue to rely on current treatment strategies that emphasize nephron-sparing therapies for active treatment candidates, and active surveillance for older patients who are poor candidates for surgery.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913-24.
2. Kato M, Suzuki T, Suzuki Y, et al. Natural history of small renal cell carcinoma: evaluation of growth rate, histological grade, cell proliferation and apoptosis. J Urol. 2004;172:863-6.
3. Tsui KH, Shvarts O, Smith RB, et al. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163:426-30.
4. Kocher KE, Meurer WJ, Fazel R, et al. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58:452-62.e3.
5. O’Connor SD, Pickhardt PJ, Kim DH, et al. Incidental finding of renal masses at unenhanced CT: prevalence and analysis of features for guiding management. AJR Am J Roentgenol. 2011;197:139-45.
6. Akdogan B, Gudeloglu A, Inci K, et al. Prevalence and predictors of benign lesions in renal masses smaller than 7 cm presumed to be renal cell carcinoma. Clin Genitourin Cancer. 2012;10:121-5.
7. Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271-9.
8. Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203-5.
9. Tomaszewski JJ, Smaldone MC, Uzzo RG, Kutikov A. Is radical nephrectomy a legitimate therapeutic option in patients with renal masses amenable to nephron-sparing surgery? BJU Int. 2015;115:357-63.
10. Lee H, Lee JK, Kim K, et al. Risk of metastasis for T1a renal cell carcinoma. World J Urol. 2016;34:553-9.
11. Shih YC, Chien CR, Xu Y, et al. Economic burden of renal cell carcinoma in the US: Part II--an updated analysis. Pharmacoeconomics. 2011;29:331-41.
12. Asnis-Alibozek AG, Fine MJ, Russo P, et al. Cost of care for malignant and benign renal masses. Am J Manag Care. 2013;19:617-24.
13. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151-9.
14. Birnbaum BA, Hindman N, Lee J, Babb JS. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology. 2007;244:767-75.
15. Vargas HA, Chaim J, Lefkowitz RA, et al. Renal cortical tumors: use of multiphasic contrast-enhanced MR imaging to differentiate benign and malignant histologic subtypes. Radiology. 2012;264:779-88.
16. Gorin MA, Rowe SP, Baras AS, et al. Prospective evaluation of (99m) Tc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol. 2016;69:413-6.
17. Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol. 2007;8:304-10.
18. Muselaers CH, Rijpkema M, Bos DL, et al. Radionuclide and fluorescence imaging of clear cell renal cell carcinoma using dual labeled anti-carbonic anhydrase IX antibody G250. J Urol. 2015;194:532-8.
19. Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol. 2014;31:20-6.
20. Richard PO, Jewett MA, Bhatt JR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68:1007-13.
21. Mullins JK, Rodriguez R. Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J. 2013;7:E176-E179.
22. Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60:578-84.
23. Rahbar H, Bhayani S, Stifelman M, et al. Evaluation of renal mass biopsy risk stratification algorithm for robotic partial nephrectomy--could a biopsy have guided management? J Urol. 2014;192:1337-42.
24. Richard PO, Jewett MA, Bhatt JR, et al. Active surveillance for renal neoplasms with oncocytic features is safe. J Urol. 2016;195:581-7.
25. Su LM, Kuo J, Allan RW, et al. Fiber-optic confocal laser endomicroscopy of small renal masses: toward real-time optical diagnostic biopsy. J Urol. 2016;195:486-92.
26. Turner RM. How renal mass biopsy impacts management. Urology Times. March 14, 2016. http://urologytimes.modernmedicine.com/urology-times/news/how-renal-mass-biopsy-impacts-management. Accessed May 6, 2016.
27. Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39-44.
28. Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015;68:408-15.
29. Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118:997-1006.
30. Bhan SN, Pautler SE, Shayegan B, et al. Active surveillance, radiofrequency ablation, or cryoablation for the nonsurgical management of a small renal mass: a cost-utility analysis. Ann Surg Oncol. 2013;20:3675-84.
31. Pandharipande PV, Gervais DA, Hartman RI, et al. Renal mass biopsy to guide treatment decisions for small incidental renal tumors: a cost-effectiveness analysis. Radiology. 2010;256:836-46.
32. Herr HW. A history of partial nephrectomy for renal tumors. J Urol. 2005;173:705-8.
33. Nieder AM, Taneja SS. The role of partial nephrectomy for renal cell carcinoma in contemporary practice. Urol Clin North Am. 2003;30:529-42.
34. Poulakis V, Witzsch U, de Vries R, et al. Quality of life after surgery for localized renal cell carcinoma: comparison between radical nephrectomy and nephron-sparing surgery. Urology. 2003;62:814-20.
35. Van Poppel H, Joniau S. Is surveillance an option for the treatment of small renal masses? Eur Urol. 2007;52:1323-30.
36. Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468-71.
37. Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55-61.
38. Thompson RH, Lane BR, Lohse CM, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012;79:356-60.
39. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543-52.
40. Liss MA, Wang S, Palazzi K, et al. Evaluation of national trends in the utilization of partial nephrectomy in relation to the publication of the American Urologic Association guidelines for the management of clinical T1 renal masses. BMC Urol. 2014;14:101.
41. Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41-6.
42. Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol. 2010;183:473-9.
43. Gong EM, Orvieto MA, Zorn KC, et al. Comparison of laparoscopic and open partial nephrectomy in clinical T1a renal tumors. J Endourol. 2008;22:953-7.
44. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844-53.
45. Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786-93.
46. Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method. J Urol. 2010;183:1708-13.
47. Simmons MN, Hillyer SP, Lee BH, et al. Nephrometry score is associated with volume loss and functional recovery after partial nephrectomy. J Urol. 2012;188:39-44.
48. Spaliviero M, Poon BY, Karlo CA, et al. An arterial based complexity (ABC) scoring system to assess the morbidity profile of partial nephrectomy. Eur Urol. 2016;69:72-9.
49. Mir MC, Campbell RA, Sharma N, et al. Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology. 2013;82:263-8.
50. Castaneda CV, Danzig MR, Finkelstein JB, et al. The natural history of renal functional decline in patients undergoing surveillance in the DISSRM registry. Urol Oncol. 2015;33:166.e17-20.
51. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-305.
52. Zargar H, Allaf ME, Bhayani S, et al. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int. 2015;116:407-14.
53. Welch BT, Brinjikji W, Schmit GD, et al. Evaluation of the charges, safety, and mortality of percutaneous renal thermal ablation using the nationwide inpatient sample. J Vasc Interv Radiol. 2015;26:342-7.
54. Lian H, Guo H, Zhang G, et al. Single-center comparison of complications in laparoscopic and percutaneous radiofrequency ablation with ultrasound guidance for renal tumors. Urology. 2012;80:119-24.
55. Young EE, Castle SM, Gorbatiy V, Leveillee RJ. Comparison of safety, renal function outcomes and efficacy of laparoscopic and percutaneous radio frequency ablation of renal masses. J Urol. 2012;187:1177-82.
56. Kim SD, Yoon SG, Sung GT. Radiofrequency ablation of renal tumors: four-year follow-up results in 47 patients. Korean J Radiol. 2012;13:625-33.
57. Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2013;200:461-6.
58. Samarasekera D, Khalifeh A, Autorino R, Kaouk J. Percutaneous radiofrequency ablation versus percutaneous cryoablation: long-term outcomes following ablation for renal cell carcinoma. J Urol. 2013;189:e737-e738. Abstr 1795.
59. Zargar H, Atwell TD, Cadeddu JA, et al. Cryoablation for small renal masses: selection criteria, complications, and functional and oncologic results. Eur Urol. 2016;69:116-28.
60. Whitson JM, Harris CR, Meng MV. Population-based comparative effectiveness of nephron-sparing surgery vs ablation for small renal masses. BJU Int. 2012;110:1438-43.
61. Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486-92.
62. de Senneville BD, Moonen C, Ries M. MRI-guided HIFU methods for the ablation of liver and renal cancers. Adv Exp Med Biol. 2016;880:43-63.
63. Hernandez JI, Cepeda MF, Valdes F, Guerrero GD. Microwave ablation: state-of-the-art review. Onco Targets Ther. 2015;8:1627-32.
64. Siva S, Ellis RJ, Ponsky L, et al. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol. 2016;12:637-45.
65. Narayanan G, Doshi MH. Irreversible electroporation (IRE) in renal tumors. Curr Urol Rep. 2016;17:15.
66. Lane BR, Demirjian S, Derweesh IH, et al. Survival and functional stability in chronic kidney disease due to surgical removal of nephrons: importance of the new baseline glomerular filtration rate. Eur Urol. 2015;68:996-1003.
67. Lane BR, Campbell SC, Demirjian S, Fergany AF. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189:1649-55.
68. Castle SM, Gorbatiy V, Avallone MA, et al. Cost comparison of nephron-sparing treatments for cT1a renal masses. Urol Oncol. 2013;31:1327-32.
69. Kowalczyk KJ, Choueiri TK, Hevelone ND, et al. Comparative effectiveness, costs and trends in treatment of small renal masses from 2005 to 2007. BJU Int. 2013;112:E273-E280.
70. Morrissey JJ, Mellnick VM, Luo J, et al. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: a prospective cohort study. JAMA Oncol. 2015;1:204-12.
71. Nayan M, Richard PO, Jewett MA, et al. Hematologic parameters to predict small renal mass biopsy pathology. Clin Genitourin Cancer. 2015 Dec 17. [Epub ahead of print]
72. Christinat Y, Krek W. Integrated genomic analysis identifies subclasses and prognosis signatures of kidney cancer. Oncotarget. 2015;6:10521-31.
73. Iliev R, Stanik M, Fedorko M, et al. Decreased expression levels of PIWIL1, PIWIL2, and PIWIL4 are associated with worse survival in renal cell carcinoma patients. Onco Targets Ther. 2016;9:217-22.
74. Le Tourneau C, Kamal M, Tsimberidou AM, et al. Treatment algorithms based on tumor molecular profiling: the essence of precision medicine trials. J Natl Cancer Inst. 2016;108:pii:djv362.
75. Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-20.
76. Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182:41-5.
77. Wang S, Qin C, Peng Z, et al. Radiofrequency ablation versus partial nephrectomy for the treatment of clinical stage 1 renal masses: a systematic review and meta-analysis. Chin Med J (Engl). 2013;127:2497-503.