Current Issues in Lung Cancer Screening
In this review we will discuss issues inherent to the lung cancer screening process, including the value of smoking cessation strategies, the challenge with the rapid pace of developments in the field, cost concerns, consideration of biases in trial design (overdiagnosis, for example), overtreatment, and radiation risk. We discuss recommendations from several organizations, such as the US Preventive Services Task Force and the American Cancer Society.
Despite improvements in drug therapy, late-stage lung cancer remains routinely incurable. The field of screening for early lung cancer is a challenging, fast-moving, cross-disciplinary area, not without controversy. The ideal situation is one in which we approach screening proactively to allow it to mature as a public health strategy. Spiral CT screening for lung cancer is a new and promising approach to thoracic imaging; it allows for a much more rapid and comprehensive evaluation of a structure than the original CT scan. In this review we will discuss issues inherent to the lung cancer screening process, including the value of smoking cessation strategies, the challenge with the rapid pace of developments in the field, cost concerns, consideration of biases in trial design (overdiagnosis, for example), overtreatment, and radiation risk. We discuss recommendations from several organizations, such as the US Preventive Services Task Force and the American Cancer Society.
Lung cancer has killed over 1 million victims in this country over the past 5 years and will kill at least that many over the next 5 years as well.[1-3] The overwhelming majority of these victims die due to a late diagnosis. Chest x-ray is a poor tool for finding early curable disease. Despite improvements in drug therapy, late-stage lung cancer is routinely incurable. There have been a number of detailed reviews on the subject of lung cancer screening recently published in highly visible journals. As recently reviewed by the United States Preventive Services Task Force (USPSTF), lung cancer screening is a dynamic area where recent publications have led to a change in their recommendation from discouraging lung cancer screening to an I classification:
"The USPSTF makes no recommendation (either for or against) the use of computed tomography (CT) in persons who have no symptoms of lung cancer. If screening is being considered, physicians are advised to discuss with the patient the pros and cons, with an emphasis on the lack of studies showing that screening helps people live longer and on reports that false positive test results are common and can lead to unnecessary worry, testing, and surgery."[4]
This indeterminate designation, which is the same classification as for prostate cancer screening with prostate-specific antigen, is based on a lack of definitive data for or against screening.
In the course of this review, issues inherent to the lung cancer screening process will be discussed, considering how each step could be optimized to enhance prospects for eventual screening benefit. This is a challenging, fast-moving, and cross-disciplinary area. It is important that oncologists have in-depth exposure to the spectrum of such issues to inform their clinical actions and discussions with patients in this area.
Spiral CT screening for lung cancer is a new and promising approach to thoracic imaging. Spiral CT differs from the original CT scan related to the design of the scanner; it allows much more rapid and comprehensive evaluation of a structure.[5] Even spiral CT imaging has undergone considerable improvement even since the first positive pilot articles on lung cancer screening with spiral CT in 1999, when Henschke and coworkers first reported their promising results with this tool.[6] In addition, there have been improvements in software tools and many other related aspects of lung cancer screening.[5,7]
As we and several others have recently discussed, there are still many significant challenges in learning how to most efficiently, effectively, and economically provide this service. The dominant public health response to the high rate of lung cancer mortality has been to use tobacco control strategies to address the problem of lung cancer. In both the Mayo Clinic and Cornell CT screening studies, smoking cessation counseling in the setting of lung cancer screening was associated with more favorable quit rates.[8,9]
While smoking cessation strategies are of fundamental importance in rapidly improving outcomes in cardiovascular disease, the risk of developing lung cancer remains elevated after smoking cessation.[10] Lung cancers are being diagnosed at least as frequently in the over 45 million former smokers as in current smokers; smoking cessation strategies are of no further utility in that growing cohort of former smokers.[11]
As progress in cardiovascular disease has not been matched in lung cancer outcomes, the result is that lung cancer has recently emerged as the dominant cause of death in tobacco-exposed individuals.[12] Tobacco-related diseases are the leading cause of premature death and account for half of health-care costs in our society, so better approaches to lung cancer management beyond smoking cessation are critical at least for the one in four adults in our society who are currently former smokers.[13] Recently, thoughtful publications have called for a full-scale reconsideration of our efforts to significantly impact unfavorable cancer outcomes.[ 14] Implementing a much more comprehensive and carefully organized program of early lung cancer detection and management research is the most promising way to improve outcomes in this broad segment of our society.[15]
Where Are We Now With Lung Cancer Outcomes?
With over 160,000 deaths projected for this year, lung cancer accounts for 30% of cancer deaths in the United States.[1] Regional or distant metastatic spread is evident in at least three-quarters of lung cancer cases at time of diagnosis, so the 5-year survival rate for lung cancer is about 15%. For localized cancer, the 5-year survival rates are often better than 60%. As a point of reference, localized breast and prostate cancer are detected at rates of 63% and 82%, with 5-year survival rates of 87% and 98%, respectively.
Promising reports with highresolution CT detection have renewed interest in early lung cancer screening but no major lung cancer screening trial has been completed in the United States since the early 1980s. There is growing appreciation of the methodologic limitations of the previous chest x-ray screening trials and their inadequacy as a basis for current health-care policy.[4] However, concern about the cost and other healthcare consequences of CT-based lung cancer detection has led to considerable controversy about the merits of this direction. Against this charged backdrop, it is timely to review the status of early lung cancer detection.
All single-arm, spiral CT screening studies have reported considerably higher stage I frequency than the national experience of 17%, with some studies reporting stage I detection frequency in excess of 80%.[7,16] From a large Japanese experience from 1975 to 1993, 26,338 screening chest xrays detected 42% of cases as stage I lung cancer with an average primary size of 3 cm; 33% were stage III/ IV.[17] During 1993, this group began using CT; by 2002, 15,342 scans were performed. With CT screening, 78% of the detected cases were stage I with a mean diameter of 1.5 cm and the rate of detection for stage III/VI disease had decreased to 14%. With this transition, the overall 5-year survival improved from 49% with chest x-ray-detected cases to 84% with CTdetected cases. This result is not definitive, as the observational study design cannot account for all sources of potential bias in the study subject. Yet it still constitutes a remarkable improvement in outcomes compared to historical standards. Other Japanese groups have reported similar positive experiences.[18]
The International Early Lung Cancer Action Project (I-ELCAP) screening experience with current and former smokers was recently presented with prevalence evaluation of over 26,000 subjects and follow-up incidence data from 19,700 subjects.[19,20]. In this multinational experience with over 350 lung cancers detected, the frequency of stage I was 82%. With follow-up on some cases as far out as 100 months, the lung cancer-related survival rate was over 95%.
The notable factor associated with this remarkable experience is that the I-ELCAP study sites adhere to a standardized multidisciplinary algorithm for case workup and therapeutic intervention. From their long experience with lung cancer screening at the Weill Medical College of Cornell University, they have evolved to a defined best practice for all aspects of the lung cancer screening process.[ 18,19] In the process they have redefined the clinical management of early lung cancer. They have approached this challenge as a public health issue, looking at factors such as optimizing cost.[20] To accomplish this, they have pioneered the development of image processing tools to use the dynamic information extractable from serial high-resolution spiral CT as a reflection of biological aggressiveness to segregate clinically significant lung cancer nodules.[21-23] They have evolved a much more disciplined and minimally invasive approach to case workup.[24] They have defined a new lexicon of terms to define a phase of early lung cancer that was clinically inapparent prior to the advent of highresolution spiral CT.[25]
The rapid pace and scope of this work has been disruptive in the field, stirring controversy.[26] The literature in the field has been confusing to many, as issues such as cost efficiency have been so widely divergent.[ 20,26] This dynamic tension is heightened by the amount and intensity of public interest in this area, leaving the practicing oncologist attempting to respond to inquiries from patients in a quandary. A major concern about widespread CT screening relates to its cost, especially in light of one study that projected enormous costs from models assembled using assumptions based on early screening reports. [27] In contrast, more extensive use of noninvasive imaging techniques in the workup of screen-detect lesions may explain why the cost features of other screening management approaches are less expensive. With the ELCAP approach only 13% of the screened cases require further follow-up, with most of those cases evaluated by serial CT imaging for nodule growth rate.[23-25] Further potential for cost savings and morbidity reductions can be achieved by carefully defining the risk features of the screened cohort, by reducing the screening intensity in following up screen-negative populations, as well as by furthering innovation with the imaging technology.
Definitive Evaluation of a Cancer Screening Effect
The traditional end point of a randomized trial for success of a screening modality is a significant cancer-related mortality reduction in the screened population compared to the control population. The randomization of the subjects entering on the trial is to address the potentially confounding influence of biases, in particular, overdiagnosis, as recently reviewed.[7] The term "overdiagnosis" refers to clinical outcome events not adjusted for disease that would remain clinically covert until death from other causes. If there is considerable overdiagnosis, an apparently favorable screening result in regard to stage or 5-year survival would not lead to a significant lung cancer mortality reduction in the screened arm. With current information it is not possible to establish a reliable estimate of the magnitude of overdiagnosis, but emerging clinical and biological information suggest that these small screen-detected lung cancers appear to behave like symptom-detected lung cancers.[28,29]
Furthermore, the term overdiagnosis is used loosely and may be construed to include the situation where a clinically aggressive lung cancer is detected by CT screening but the patient expires first of a comorbid condition. In the decades since the last major National Cancer Institute (NCI)-sponsored lung cancer screening trials using chest x-ray, the influence of competing risks has diminished related to both improved coronary artery disease outcomes and the increasing number of former smokers; this has perhaps mitigated the influence of overdiagnosis.[7,11,13] Finally, overdiagnosis could also be construed as cases where lethal iatrogenic complications occur in the course of clinical management of screen-detected lesions.[ 26]
A more important factor to consider in regard to lung cancer screening is the concept of overtreatment. Overtreatment refers to the use of an intervention that may entail greater morbidity than necessary to accomplish a clinical goal. The ultimate goal of a cancer screening process is to develop the least intrusive and risky way to avoid high-risk subjects going from progressive to lethal metastatic disease.
While there are many potential risks with lung cancer screening management, some of these risks may be overstated in the literature. A recent report on radiation risk reported the potential frequency of eventual carcinogenesis associated with low-dose spiral CT based on highly conservative extrapolations using our existing but limited human radiation exposure information.[30] In the typical older, heavily tobacco-exposed individual, the risk of lung cancer is higher by orders of magnitude than the theoretical risk of a radiation-induced cancer in a geriatric at-risk population. While prudence dictates minimal exposure to radiation, the cost-benefit analysis in the setting of heavily exposed target screening populations is heavily weighted by the well-documented lethality of tobacco exposure. This issue bears discussion, especially with younger individuals, as part of the informed consent process around lung cancer screening.
A randomized trial is supposed to test two stable options for clinical management, but there are a number of methodologic limitations in using this clinical trial tool for evaluation of screening.[31,32] Of concern with lung cancer screening is the fact that the imaging technology with spiral CT is improving so rapidly that the type of lung cancer screening used at the beginning of the screening trial may already be obsolete within the decade it takes to complete a typical randomized screening trial. This rapid pace of technologic innovation, with lung cancer imaging doubling in resolution every 2 years, is an unprecedented challenge to the legitimacy of the randomized trial in this setting.[7]
The challenge of cancer screening evaluation is further and profoundly complicated by the rapid introduction of new detection technology in our market-driven health-care system.[33] Consequently, there is a pressure to evaluate new detection technology in advance of formal validation of such a technology. But the downside of this approach is that the nuances of clinical integration of such a detection tool may not yet be defined. Further, if a definitive evaluation trial cannot be completed within a relevant time interval for a technology, there is a profound problem with using such a clinical evaluation strategy. Therefore, while this challenge has emerged first with spiral CT for lung cancer screening, given the pace of imaging research in general, this situation may be encountered with other imaging modalities in the future. It deserves serious thought from the public health research community.
Every major improvement in the resolution of lung cancer imaging will also affect the downstream clinical management of the type of disease now detected by the more capable imaging. For example, as suggested by Japanese surgical parties, the best clinical management for small CT-detected primary cancers is likely to be different than required for bulkier chest x-ray-detected lung cancers.[34-36] In the setting of annual screening, where the detected primary cancer is likely to be under a centimeter in diameter, for example, is an anatomic lobectomy with mediastinal dissection the appropriate operation to remove a 7-mm peripheral primary lung cancer? However, even with subcentimeter screen-detected primary cancers, the frequency of detecting lung cancer beyond stage I remains around 10%, so the optimal size range for reliably finding only localized stage I cancers is not yet known. Defining less aggressive intervention techniques to avoid iatrogenic complications in this screening setting is an important goal of early-detection research.[37]
From the experience of a number of centers of excellence from three continents, it is clear that high-quality lung cancer screening care and measures reducing the number of invasive diagnostic procedures can improve cost efficiencies.[7] Professional groups such as the Society for Thoracic Surgery have developed national registries as a tool for improving quality outcomes in thoracic surgery (http://www.sts.org/doc/ 8406); these measures may allow favorable management outcomes to become more generalized. Currently, the best approach to reducing overtreatment and with it the morbidity and mortality of screening case management is an area in which clear research opportunities exist. Preliminary studies in this regard are under way.
In light of the reports suggesting spiral CT can detect small, early lung cancer, the NCI rapidly initiated the National Lung Cancer Screening Trial (NLST) to evaluate if CT screening leads to a significant improvement in lung cancer-related mortality. This urgency was heightened by the concern that widespread ad hoc CT screening was occurring even though the cost of the initial CT was not being reimbursed.
The NLST, which has already completed full accrual, uses multi-detectorrow scanners (mostly four rows) for the 25,000 volunteers on the CT arm of that trial. The control group of 25,000 receives annual chest x-ray screening. The NLST subjects will receive annual screening for 3 years, and follow-up will continue for a few years until a mortality end point is reached.[7] The Dutch national randomized CT screening trial will use 16-detector scanners and computerassisted detection (CAD) tools for their entire study population and compare outcomes with a standard care control arm using a carefully defined high-risk population.[38] Other European trials, including studies in France and Italy, are coming on line. As supported by the American Cancer Society (ACS) and the NCI, investigators from the US and European trials will have periodic meetings to standardize elements of data acquisition so that comparison of results from the various trials may be more productive.
While breast cancer screening trials were conducted over several decades with relative stability of the imaging detection tool,[39] this has not been the case with spiral CT. Yet the experience with breast cancer screening research is still instructive to lung cancer on many levels.[40-45]
Rapidly Changing Lung Cancer Imaging Technology
A major difference between lung cancer and breast imaging is the fact that over the past decade, the substantial improvements in the speed and quality of CT imaging have allowed for much more realistic three-dimensional representation of actual respiratory anatomy.[46,47] While CAD has not had a major clinical impact on breast cancer imaging,[45] this situation could be different for lung cancer because the anatomical relationships are not distorted by breast compression and two-dimensional image acquisition constraints.
With the latest 64-detector-row scanners imaging the entire thorax with less than 0.625-mm slice thickness in several seconds, the amount of data generated in this process is daunting. Currently, the average size of incident primary cancers detected at one center is under 1.0 cm. The gap between the technical capabilities of the hardware in acquiring vast amounts of imaging data and the availability of validated software to harness this improved imaging capability highlights the importance of research into CAD for early cancers. A potential benefit of higher-resolution imaging is that the evaluation may be more sensitive and further decrease the frequency of interval-detected cancer.
If CT screening is validated to be effective, many more lung CT scans will be performed. Even with screening high-risk cohorts, the frequency of cancer in a high=risk population will typically be about 1% or less, so software to allow efficient work flow is essential to leveraging the productivity of thoracic radiologists. However, to reliably establish clinically relevant features such as the irregular boundary of small pulmonary lesions abutting normal adjacent structures, the amount of imaging information required by a CAD system may exceed the amount of imaging information that is reasonable to expect a radiologist to review. This challenge will be most evident when CAD is being applied to evaluate very small lesions (under 6 mm), where human vision has limited capabilities and determining "ground truth" will be problematic.
Therefore, developing and validating CAD applications for cancer screening are great challenges, but standardized image evaluation tools may prove essential in moving population-based lung cancer screening into routine care settings. For this reason, the NCI developed the Lung Image Database Consortium (LIDC) to accelerate the maturation of image processing tools for CAD. The key aspect of this cooperative group is to create a large, well-characterized database of images and clinical outcomes data for CAD algorithm research and validation.[48] This resource could expedite such projects as the utility of volumetrically determined growth rates for identifying potential cancerous pulmonary nodules or studies on the natural history of newly reported ground-glass opacities (or nonsolid nodules).
FIGURE 1
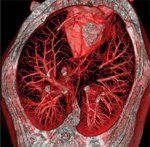
Rendering of a Volumetric CT Scan
Rapid progress with CT-based imaging is expected to continue. An example of state-of-the-art CT imaging is shown in Figure 1: an image of dog lung is shown that was acquired on a CT imaging prototype, being developed by General Electric Global Research Center, that is capable of imaging the thorax and producing volumetric images with spatial resolution that is eight times higher than state-of-the-art clinical CT systems. To extract clinically significant information from such a detail-rich image, computer-assisted tools will be crucial. The pragmatic position is shaped by the challenges encountered in breast cancer screening efforts in regard to radiologists' workload, reimbursement, and professional liability.[40-45] From this much more mature image-based screening experience, it is clear that many developmental problems for thoracic radiologists may be ameliorated if validated computer-aided diagnosis is rapidly developed.
Conclusion: What Should Be Discussed With a Patient Considering Screening?
TABLE 1

Points to Consider When Discussing Lung Cancer Screening With Individuals Considering Spiral CT Screening in Advance of Validated Evidence
In a recent New England Journal of Medicine article, we proposed a list of issues to consider in discussion with a patient that may be considering lung cancer screening (Table 1).[7] Progressively professional societies are delegating to the clinician the responsibility for communicating to patients the merits and limitations of such complex and dynamic topics. In light of recent reports about health literacy and patient perception, there is also a major communications challenge in assisting clinicians in their dialogues with patients on such complex issues (http://www.iom.edu/report.asp?id=19723 and http://www.ahrq.gov/clinic/epcsums/ litsum.htm).
Because the clinical management for lung cancer screening has a higher probability of morbid and mortal complications than cancer screening for other organs, a mortality reduction benefit found by the NLST may not result in improved national outcomes with lung cancer if high-quality screening care delivery systems for early lung cancer are not in place.[15] The choice is between organized screenings, where screening services are provided in centers committed to excellence in early cancer management, or ad hoc screening, where the specifics of screening care are left to be refined by market forces.[49]
The ACS updated its statement on testing for early lung cancer while recommending against testing for early lung cancer detection in the asymptomatic population of at-risk individuals. Their revised statement recommends that individuals at high risk for lung cancer, due to significant exposure to tobacco smoke or occupational exposure, should discuss with their physician the potential benefits and harms to inform their testing decision. However, the ACS recommends that for individuals seeking screening services, downstream clinical management should be done only in experienced centers linked to multidisciplinary specialty groups for diagnosis and follow-up.
So while the USPSTF statement represents a change from their previous recommendation against screening, and this reflects the accumulation of more persuasive though not yet definitive data regarding the utility of lung cancer screening, considerably more research is needed in this area to fully exploit the capability of spiral CT to visualize preinvasive lung cancer. Early detection of lung cancer with spiral CT clearly represents the most significant opportunity to improve cancer mortality outcomes. For this reason, it is critical to take this opportunity seriously.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Peto R, Chen ZM, Boreham J: Tobacco- The growing epidemic. Nat Med 5:15-17, 1999.
2. Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004.
3. Patel JD, Bach PB, Kris MG: Lung cancer in US women: A contemporary epidemic. JAMA 291:1763-1768, 2004.
4. Humphrey LL, Teutsch S, Johnson M: Lung cancer screening with sputum cytological examination, chest radiography and computer tomography: An update for the U.S. Preventive Services Task Force. Ann Intern Med 140:740-753, 2004.
5. Mulshine J: Screening for lung cancer: In pursuit of pre-metastatic disease. Nat Rev Cancer 3:65-73, 2003.
6. Henschke CI, McCauley DI, Yankelevitz DF, et al: Early Lung Cancer Action Project: Overall design and findings from baseline screening. Lancet 354:99-105, 1999.
7. Mulshine JL, Sullivan D: Lung cancer screening. N Engl J Med 352:42-48, 2005.
8. Ostroff JS, Buckshee N, Mancuso CA, et al: Smoking cessation following CT screening for early detection of lung cancer. Prev Med 33:613-621, 2001.
9. Cox LS, Clark MM, Jett JR, et al: Change in smoking status after spiral chest computed tomography scan screening. Cancer 98:2495- 2501, 2003.
10. Enstrom JE, Heath CW, Jr: Smoking cessation and mortality trends among 118,000 Californians, 1960-1997. Epidemiology 10:500-512, 1999.
11. Tong L, Spitz MR, Fueger JJ, et al: Lung carcinoma in former smokers. Cancer 78:1004- 1010, 1996.
12. Centers for Disease Control and Prevention: Annual smoking-attributable mortality, years of potential life lost and economic cost- United States 1995-1999. Morb Mortal Wkly Rep 51:300-303, 2002.
13. Lenfant C: Shattuck lecture: Clinical research to clinical practice-Lost in translation? N Engl J Med 349:868-874, 2003.
14. Leaf C: Why we’re losing the war on cancer and how to win it. Fortune 76-96, 2004.
15. Warner EE, Mulshine JL: Lung cancer screening with spiral CT: Toward a working strategy. Oncology 18:564-575, 2004.
16. Henschke CI, Yankelevitz DF, McCauley DI, et al: Guidelines for the use of spiral computed tomography in screening for lung cancer. Eur Respir J Suppl 39:45s-51s, 2003.
17. Kakinuma R: Low-dose helical CT screening for lung cancer: The Japanese experience and perspective. Proceedings of the International Association for the Study of Lung Cancer Workshop 18:2003.
18. Kaneko M, Kusumoto M, Kobayashi T, et al: Computed tomography screening for lung carcinoma in Japan. Cancer 89:2485-2488, 2000.
19. Henschke CI, Yankelevitz D: Lung cancer screening with spiral CT: Toward a working strategy (review). Oncology 18:584-587, 2004.
20. Wisnivesky JP, Mushlin AI, Sicherman N, et al: The cost-effectiveness of low-dose CT screening for lung cancer: Preliminary results of baseline screening. Chest 124:614-621, 2003.
21. Kostis WJ, Reeves AP, Yankelevitz DF, et al: Three-dimensional segmentation and growth-rate estimation of small pulmonary nodules in helical CT images. IEEE Trans Med Imaging 22:1259-1274, 2003.
22. Kostis WJ, Yankelevitz DF, Reeves AP, et al: Small pulmonary nodules: Reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology 231:446-452, 2004.
23. Yankelevitz DF, Reeves AP, Kostis WJ, et al: Small pulmonary nodules: Volumetrically determined growth rates based on CT evaluation. Radiology 217:251-256, 2000.
24. Libby DM, Smith JP, Altorki NK, et al: Managing the small pulmonary nodule discovered by CT. Chest 125:1522-1529, 2004.
25. Henschke CI, Yankelevitz DF, Mirtcheva R, et al: CT screening for lung cancer: Frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 178:1053-1057, 2002.
26. Swensen SJ, Jett JR, Midthun DE, et al: computer tomographic screening for lung cancer: Home run or foul ball? Mayo Clin Proc 78:1187-1188, 2003.
27. Mahadevia PJ, Fleisher LA, Frick KD, et al: Lung cancer screening with helical computed tomography in older adult smokers: A decision and cost-effectiveness analysis. JAMA 289:313-322, 2003.
28. Bianchi F, Hu J, Pelosi G, et al: Lung cancers detected by screening with spiral computed tomography have a malignant phenotype when analyzed by cDNA microarray. Clin Cancer Res 10:6023-6028, 2004.
29. Mulshine JL, Weinstein JN: Is the gene expression pattern different in lung cancer detected by screening spiral CT rather due to symptoms? Clin Cancer Res 10:5973-5974, 2004.
30. Brenner DJ: Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 231:440- 445, 2004.
31. Sackett DL, Wennberg JE: Choosing the best research design for each question. BMJ 315:1636, 1997.
32. Concato J, Shah N, Horwitz RI: Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342:1887-1892, 2000.
33. Smith RA, Cokkinides V, Eyre HJ: American Cancer Society Guidelines for the Early Detection of Cancer, 2003. CA Cancer J Clin 53:27-43, 2003.
34. Fang D, Zhang D, Huang G, et al: Results of surgical resection of patients with primary lung cancer: A retrospective analysis of 1,905 cases. Ann Thorac Surg 72:1155-1159, 2001.
35. Konaka C, Ikeda N, Hiyoshi T, et al: Peripheral non-small cell lung cancers 2.0 cm or less in diameter: Proposed criteria for limited pulmonary resection based upon clinicopathological presentation. Lung Cancer 21:185-191, 1998.
36. Asamura H, Suzuki K, Watanabe S, et al: A clinicopathological study of resected subcentimeter lung cancers: A favorable prognosis for ground glass opacity lesions. Ann Thorac Surg 76:1016-1022, 2003.
37. Warner EE, Mulshine JL: Surgical considerations with lung cancer screening. J Surg Oncol 84:1-6, 2003.
38. van Klaveren RJ, de Koning HJ, Mulshine J, et al: Lung cancer screening by spiral CT. What is the optimal target population for screening trials? Lung Cancer 38:243- 252, 2002.
39. Shapiro S: Screening: Assessment of current studies. Cancer 74:231-238, 1994.
40. Beam CA, Layde PM, Sullivan DC: Variability in the interpretation of screening mammograms by US radiologists. Findings from a national sample. Arch Intern Med 156:209-213, 1996.
41. Elmore JG, Carney PA: Computer-aided detection of breast cancer: Has promise outstripped performance? J Natl Cancer Inst 96:162-163, 2004.
42. Bassett LW, Monsees BS, Smith RA, et al: Survey of radiology residents: Breast imaging training and attitudes. Radiology 227:862-869, 2003.
43. Enzmann DR, Anglada PM, Haviley C, et al: Providing professional mammography services: Financial analysis. Radiology 219:467-473, 2001.
44. Kopans DB: Mammography screening is saving thousands of lives, but will it survive medical malpractice? Radiology 230:20-24, 2004.
45. Gur D, Sumkin JH, Rockette HE, et al: Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst 96:185-190, 2004.
46. Wormanns D, Fiebich M, Saidi M, et al: Automatic detection of pulmonary nodules at spiral CT: Clinical application of a computeraided diagnosis system. Eur Radiol 12:1052- 1057, 2002.
47. Reeves AP, Kostis WJ: Computer-aided diagnosis for lung cancer. Radiol Clin North Am 38:497-509, 2000.
48. LP Clarke, BY Croft, E Staab, et al: National Cancer Institute initiative: Lung image database resource for imaging research. Acad Radiol 8:447-450, 2001.
49. Smith RA: Lung cancer screening with spiral CT: Toward a working strategy (review). Oncology 18:578-583, 2004.