Current Status and Future Potential of Advanced Technologies in Radiation OncologyPart 2. State of the Science by Anatomic Site
On November 30–December 2, 2006, the Radiation Research Program of the Division of Cancer Treatment and Diagnosis of the National Cancer Institute (NCI) hosted a workshop entitled “Advanced Technologies in Radiation Oncology: Evaluating the Current Status and Future Potential of Proton and Other Heavy Charged-Particle Radiation Therapy, Intensity Modulated Radiation Therapy and Stereotactic Radiation Therapy.”
ABSTRACT: In December 2006, the Radiation Research Program of the Division of Cancer Treatment and Diagnosis of the National Cancer Institute hosted a workshop intended to address current issues related to advanced radiation therapy technologies, with an eye toward (1) defining the specific toxicities that have limited the success of “conventional” radiation therapy, (2) examining the evidence from phase III studies for the improvements attributed to the advanced technologies in the treatment of several cancers commonly treated with radiation therapy, and (3) determining the opportunities and priorities for further technologic development and clinical trials. The new technologies offer substantial theoretical advantage in radiation dose distributions that, if realized in clinical practice, may help many cancer patients live longer and/or better. The precision of the advanced technologies may allow us to reduce the volume of normal tissue irradiated in the vicinity of the clinical target volume. Part 1 of this two-part article, which appeared in the March issue of ONCOLOGY, provided a general overview of the workshop discussion, focusing on the challenges posed by the new technologies and resources available or in development for meeting those challenges. This month, part 2 will outline the state of the science for each disease site.
On November 30–December 2, 2006, the Radiation Research Program of the Division of Cancer Treatment and Diagnosis of the National Cancer Institute (NCI) hosted a workshop entitled “Advanced Technologies in Radiation Oncology: Evaluating the Current Status and Future Potential of Proton and Other Heavy Charged-Particle Radiation Therapy, Intensity Modulated Radiation Therapy and Stereotactic Radiation Therapy.” In last month’s issue of ONCOLOGY, we provided a general overview of the workshop discussion, focusing on the challenges posed by the new technologies and resources available or in development for meeting those challenges. In part 2 of this report, we will outline the more specific data discussed regarding radiation therapy for different anatomic sites.
Objectives
One of the objectives of this meeting was to define the current state of the science for various disease sites, to include two major considerations:
• The rates of tumor control and toxicity following “traditional” conformal radiation therapy for some common cancers
• An examination of the evidence from phase III studies that the advanced technologies helped patients live longer and/or better (with a better quality of life).
In many instances, data in support of the latter are not yet available. The workshop participants did not interpret this as a negative, but rather an opportunity and a need for undertaking clinical trials. Indeed, an overarching conclusion was that we need robust quality assurance procedures for these advanced technologies that would, in turn, facilitate robust clinical trials. The need for incorporating quality-of-life measures, such as patient-reported outcomes and quality-adjusted life years, was emphasized. Some participants also underscored the need for exercising caution in employing these technologies outside of clinical trials at present.
This workshop report is not, however, intended to be an exhaustive review of the field. Many single-institution reports that have suggested benefit from the advanced technologies are not included herein.
Limitations of Traditional Radiation Therapy
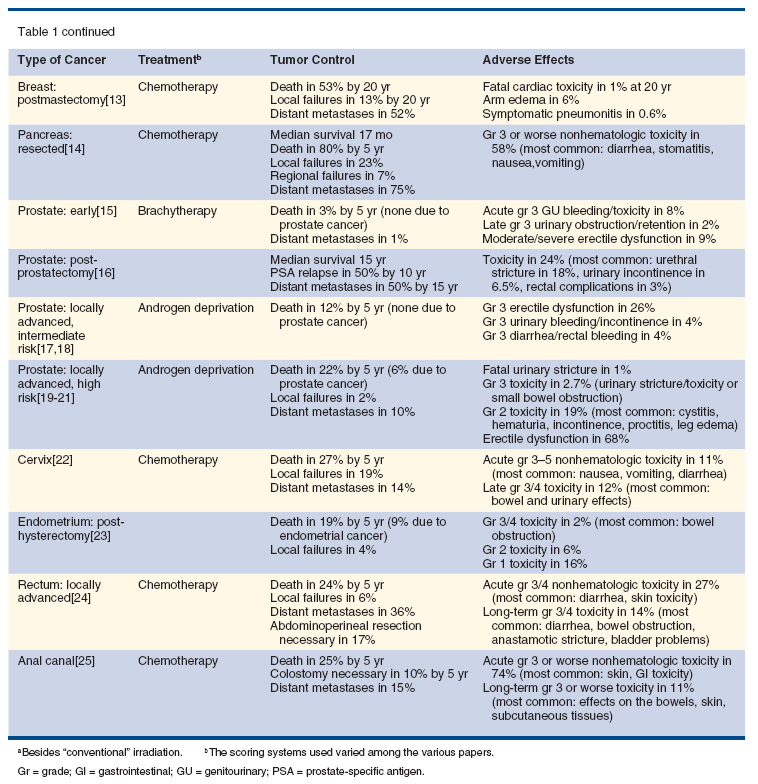
Table 1 summarizes the outcomes after conventional radiation therapy for several kinds of cancers, in terms of both tumor control and adverse effects.[2-25] While not an exhaustive list, it does illustrate-based on clinical trials that helped establish the standards of care for many common cancers-how much room there is for improvement. It also highlights what the authors of those trials reported as the most problematic adverse effects.
Evidence of Superiority of Advanced Technologies
For several disease settings-including head and neck, laryngeal, non–small-cell and small-cell lung, esophageal, pancreatic, cervical, endometrial, rectal, and anal cancers, breast cancer treated by mastectomy, and early, resected, and locally advanced intermediate-risk prostate cancer-no evidence of superiority has yet been shown for the advanced technologies in prospective randomized trials. In the following settings, some comparisons can be drawn regarding the available data for traditional and advanced radiation therapy techniques.

Single Brain Metastasis
In one arm of a prospective randomized trial,[1] traditional radiation therapy to the whole brain was delivered in 94 patients suffering from cancer and a single metastasis to the brain, between 1996 and 2001. Following the treatment, 50% of patients died within 4.9 months, and almost all died within 2 years. Local failure was observed in 29% of the treated lesions within 1 year.
Acute toxicity of grade 3 or 4 was not observed in any patient, but grade 3/4 late toxicities (occurring at or beyond 90 days) developed in 3% of patients after receiving traditional whole-brain radiation therapy. The most common side effects were nausea/vomiting, hearing loss, and central neurologic effects.
• Stereotactic Radiation Therapy-On another arm of that prospective randomized trial, 92 patients received boost irradiation by focal stereotactic radiation therapy in addition to traditional whole-brain radiation therapy. Following treatment, 50% of those patients died within 6.5 months (this duration was 1.6 months longer than in those treated without stereotactic radiation therapy, P = .039). Local failure was observed in 18% of the treated lesions within 1 year (this rate was 11% lower than in those treated without stereotactic radiation therapy, P = .01).
Acute grade 3/4 toxicities were observed in 3% (vs none among those treated without stereotactic radiation therapy), and late grade 3/4 toxicities in 6% (vs 3% without stereotactic radiation therapy) of patients receiving stereotactic radiation therapy. The early and late toxicities were, thus, slightly worse with the latter treatment but did not differ greatly between the two arms.
These results suggest that adding stereotactic radiation therapy to traditional whole-brain radiation therapy prolongs the survival of patients with single brain metastases without greatly increasing the side effects. Quality-of-life measurements and quality-adjusted life years were not reported, however. Moreover, this study did not address the question of whether delivering the boost dose of radiation by stereotactic radiation therapy was better than a boost delivered by traditional radiation therapy.
At present, there is no evidence from randomized clinical trials that the survival of patients with metastatic cancer in the brain is better, or the side effects decreased, if they are treated by intensity-modulated radiation therapy (IMRT), proton radiation therapy, or carbon-ion radiation therapy instead of traditional radiation therapy with or without stereotactic radiation therapy.
Glioblastoma
In one arm of a prospective randomized trial,[2] traditional conformal radiation therapy was delivered to 286 patients suffering from glioblastoma, between 2000 and 2002. Following treatment, 50% of patients died within 12.1 months and 90% within 2 years.
On another arm of the same study, 287 patients received temozolomide (Temodar) in addition to traditional conformal radiation therapy. Following this treatment, survival was significantly improved, with 50% of patients dying within 14.6 months and 73.5% within 2 years (P < .001).
In patients receiving radiation alone, nonhematologic grade 3/4 toxicity developed in 15%, vs 31% among those receiving radiation plus temozolomide. The most common side effects were fatigue and other constitutional symptoms, rash and other dermatologic effects, infection, effects on vision, and nausea/vomiting.
• Stereotactic Radiation Therapy-In a prospective randomized trial,[26] 203 patients with newly diagnosed glioblastoma were randomized to treatment by conventional radiation therapy (plus carmustine [BiCNU]), with vs without stereotactic radiation therapy, during 1994–2000. The results showed that the use of stereotactic radiation therapy did not improve survival, nor did it change the patterns of failure. The investigators also found no difference in the general quality of life and cognitive functioning between the two arms.
At present, there is no evidence from randomized clinical trials that the survival of patients with glioblastoma is better, or the side effects decreased, if they are treated by advanced technologies instead of or in addition to traditional conformal radiation therapy, with or without temozolomide.
Cancer of the Nasopharynx
In one arm of a prospective randomized trial,[6] traditional conformal radiation therapy (70 Gy in 1.8–2 Gy fractions over 7–8 weeks) was delivered to 92 patients suffering from nasopharyngeal cancer. Following treatment, 54% of patients died within 3 years. The patterns of failure revealed that 41% suffered local failure, while 43% developed distant metastases. On another arm of the same trial, 93 patients received chemotherapy in addition to traditional conformal radiation therapy. Following this treatment, 24% of patients died within 3 years, a significant improvement (P < .001). The patterns of failure revealed that 14% suffered local failure, whereas 15% developed distant metastases.
Of those receiving radiation alone, grade 3 or worse toxicity developed in 50%, vs 76% among those receiving the combined treatment. The most common nonhematologic side effects were stomatitis, nausea/vomiting, hearing loss, and weight loss.
• IMRT-In a prospective randomized trial,[27] 51 patients with early-stage nasopharyngeal cancer were treated by conventional radiation therapy or IMRT. The primary endpoint was the stimulated whole-salivary flow rate. The investigators hypothesized that the mean flow rate 12 months after conventional radiation therapy would be 0.05 mL/min, whereas after IMRT it would be at least 0.28 mL/min. A total of 46 patients were in remission 12 months after treatment. Their mean stimulated whole salivary flow rate was 0.05 mL/min among the controls vs 0.27 mL/min among those receiving IMRT (P < .05).
In another prospective randomized trial,[28] 60 patients with early-stage nasopharyngeal cancer were treated by conventional radiation therapy or IMRT. The primary endpoint was observer-rated xerostomia. The investigators hypothesized that 12 months after conventional radiation therapy, grade 2 or worse xerostomia would be observed in 80% of the control patients but in 40% or fewer of those receiving IMRT. Among the 58 patients still in remission 12 months after treatment, grade 2 or worse xerostomia was observed in 82.1% of the controls vs 39.3% of the IMRT recipients (P = .001). An observer-based result that close to the predicted levels may indicate observer bias, however.
These two studies suggest that when IMRT is employed for treating early-stage nasopharyngeal cancer instead of conventional radiation, the mean stimulated whole-salivary flow rate is increased while xerostomia (as assessed by the physician) is decreased.
No phase III trial has shown whether the survival of patients with nasopharyngeal cancer is better or worse if they are treated by advanced technologies instead of or in addition to traditional conformal radiation therapy, with or without cisplatin.
Early Breast Cancer Treated by Lumpectomy
In one arm of a prospective randomized trial,[12] tamoxifen plus traditional conformal radiation therapy (40 Gy in 16 fractions over 3½ weeks, followed by a 12.5-Gy boost in 5 fractions over 1 week) following lumpectomy was delivered to 386 women aged 50 years or older suffering from T1 or T2 node-negative breast cancer, between 1992 and 2000. Following the treatment, 7% of patients died within 5 years (2.5% due to breast cancer). The patterns of failure revealed that 0.6% suffered local failure within 5 years and 3.5% within 8 years, while 4.5% developed distant metastases.
The most common grade 3 side effects attributable to irradiation were fatigue in 1% and skin erythema in 1%.
• IMRT-In a prospective randomized trial,[29] 306 women with early breast cancer were randomized after lumpectomy to conventional radiation therapy (using standard wedge compensators) or IMRT between 1997 and 2000. The primary endpoint was change in breast appearance, scored from serial photographs taken before and after radiotherapy.
A total of 240 patients (79%) with 5-year photographs were available for analysis. Change in breast appearance was identified in 71 of 122 patients (58%) who were allocated conventional treatment, compared to 47 of 118 patients (40%) allocated IMRT. Patients in the control arm were 1.7 times more likely to have a change in breast appearance than those in the IMRT arm, after adjustment for year of photographic assessment (95% confidence interval = 1.2–2.5; P = .008). No significant differences between the two treatment groups were found in patient-reported breast discomfort, breast hardness, or quality of life.
In another prospective randomized trial,[30] 358 women were randomized after lumpectomy to conventional radiation therapy (using wedges) or IMRT between 2003 and 2005. It was hypothesized that 36% of patients in the control arm would suffer moderate to severe acute skin toxicity, whereas in the IMRT group, no more than 21% would. Analysis showed that 36.7% of patients in the control arm suffered such toxicity vs 27.1% in the IMRT arm. Thus, this study did not meet its primary objective. However, it was noted by the authors that among the controls, 48% developed moist desquamation vs 31% of those receiving IMRT. They also noted that women with larger breasts were more likely to suffer moist desquamation.
These two studies therefore suggest that moist desquamation in the short term (especially in large-breasted women) and a change in breast appearance in the long term are less likely among women treated by IMRT after lumpectomy. Some workshop participants objected to the use of the term “IMRT” for describing the techniques employed in those trials. They felt that-billing considerations aside-the use of multiple subfields in order to mainly improve dose heterogeneity within the target volume did not rise to the level of IMRT designed to spare the organs at risk.
As yet, no phase III trial can tell us whether the survival or local control of patients with breast cancer is better or worse, if after lumpectomy they are treated by advanced technologies instead of or in addition to traditional conformal radiation therapy.
Locally Advanced, High-Risk Prostate Cancer
In one arm of a prospective randomized trial,[19-21] traditional conformal radiation therapy (70 Gy in 35 fractions over 7 weeks) was delivered to 198 patients suffering from locally advanced prostate cancer. Following treatment, 38% of patients died within 5 years (21% due to prostate cancer). The patterns of failure revealed that 16% suffered local failure within 5 years, while 29% developed distant metastases.
On another arm of the same study, 203 patients received androgen suppression in addition to traditional conformal radiation therapy. Following treatment, 22% of patients died within 5 years (6% due to prostate cancer). The patterns of failure revealed that 2% suffered local failure, while 10% developed distant metastases.
The incidence of side effects (other than erectile dysfunction) was not different between those treated with and without androgen suppression. Fatal toxicity developed in 1% (4 patients, all of whom died due to urinary strictures). In addition, grade 3 toxicity developed in 2.7% (10 patients, including 9 with urinary strictures/toxicity and 1 with small bowel obstruction). Grade 2 toxicity developed in 19%, the most common being urinary effects (cystitis, hematuria, urinary strictures, and incontinence), proctitis, and leg edema. Erectile potency decreased in 41% of patients after treatment by radiation alone, vs 68% of patients receiving androgen suppression.
• Proton Radiotherapy-At present, no evidence from randomized clinical trials has shown that survival or side effects of patients with locally advanced prostate cancer are improved if they are treated by advanced technologies instead of or in addition to traditional conformal radiation therapy.
A prospective randomized trial[31,32] compared photon radiotherapy delivered with vs without proton radiotherapy. The investigators found no difference in survival between the two groups, but patients receiving proton radiation were more likely to suffer rectal bleeding (32% vs 12%; P = .002) and urethral strictures (19% vs 8%; P = .07). In this study, the addition of an advanced technology unexpectedly resulted in an inferior result.
Summary
The advanced technologies offer exciting and potentially substantial advantages in radiation dose distributions that may help improve patient outcomes. Many early cooperative group clinical trials involving advanced technologies primarily focused on “feasibility in the cooperative group setting” as an endpoint. We are pleased to note that since this workshop, a number of trials have incorporated specific hypotheses regarding improvements in outcomes, and some have also incorporated early stopping rules in case the outcomes prove unsatisfactory in comparison to predefined thresholds.
References:
1. Andrews DW, Scott CB, Sperduto PW, et al: Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 363:1665-1672, 2004.
2. Stupp R, Mason WP, van den Bent MJ, et al, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-996, 2005.
3. Bonner JA, Harari PM, Giralt J, et al: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567-578, 2006.
4. Cooper JS, Pajak TF, Forastiere AA, et al, Radiation Therapy Oncology Group 9501/Intergroup: Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937-1944, 2004.
5. Forastiere AA, Goepfert H, Maor M, et al: Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091-2098, 2003.
6. Al-Sarraf M, LeBlanc M, Giri PG, et al: Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol 16:1310-1317, 1998.
7. Saunders M, Dische S, Barrett A, et al: Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 52:137-148, 1999.
8. Sause W, Kolesar P, Taylor S IV, et al: Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 117:358-364, 2000.
9. Komaki R, Scott CB, Sause WT, et al: Induction cisplatin/vinblastine and irradiation vs. irradiation in unresectable squamous cell lung cancer: Failure patterns by cell type in RTOG 88-08/ECOG 4588. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys 39:537-544, 1997.
10. Turrisi AT 3rd, Kim K, Blum R, et al: Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265-271, 1999.
11. Minsky BD, Pajak TF, Ginsberg RJ, et al: INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 20:1167-1174, 2002.
12. Fyles AW, McCready DR, Manchul LA, et al: Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 351:963-970, 2004.
13. Ragaz J, Olivotto IA, Spinelli JJ, et al: Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 97:116-1126, 2005.
14. Regine WF, Winter KA, Abrams RA, et al: Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA 299:1019-1026, 2008.
15. Lawton CA, DeSilvio M, Lee WR, et al: Results of a phase II trial of transrectal ultrasound-guided permanent radioactive implantation of the prostate for definitive management of localized adenocarcinoma of the prostate (Radiation Therapy Oncology Group 98-05). Int J Radiat Oncol Biol Phys 67:39-47, 2007.
16. Thompson IM Jr, Tangen CM, Paradelo J, et al: Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized clinical trial. JAMA 296:2329-2335, 2006.
17. D’Amico AV, Manola J, Loffredo M, et al: 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 292:821-827, 2004.
18. D’Amico AV, Chen MH, Renshaw AA, et al: Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA 299:289-295, 2008.
19. Bolla M, Gonzalez D, Warde P, et al: Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 337:295-300, 1997.
20. Bolla M, Collette L, Blank L, et al: Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 360:103-108, 2002.
21. Ataman F, Zurlo A, Artignan X, et al: Late toxicity following conventional radiotherapy for prostate cancer: Analysis of the EORTC trial 22863. Eur J Cancer 40:1674-1681, 2004.
22. Morris M, Eifel PJ, Lu J, et al: Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 340:1137-1143, 1999.
23. Creutzberg CL, van Putten WL, Koper PC, et al: Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 355:1404-1411, 2000.
24. Sauer R, Becker H, Hohenberger W, et al; German Rectal Cancer Study Group: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731-1740, 2004.
25. Ajani JA, Winter KA, Gunderson LL, et al: Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA 299:1914-1921, 2008.
26. Souhami L, Seiferheld W, Brachman D, et al: Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 60:853-860, 2004.
27. Pow EH, Kwong DL, McMillan AS, et al: Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 66:981-991, 2006.
28. Kam MK, Leung SF, Zee B, et al: Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 25:4873-4879, 2007.
29. Donovan E, Bleakley N, Denholm E, et al; Breast Technology Group: Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol 82:254-64, 2007.
30. Pignol JP, Olivotto I, Rakovitch E, et al: A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 26:2085-2092, 2008.
31. Shipley WU, Verhey LJ, Munzenrider JE, et al: Advanced prostate cancer: The results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 32:3-12, 1995.
32. Gardner BG, Zietman AL, Shipley WU, et al: Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol 167:123-126, 2002.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.