Current Status of Sentinel Node Surgery in Breast Cancer
Krag and Harlow believe thatsentinel lymph node biopsyfor breast cancer is still an experimentalprocedure because “longtermrandomized trials comparing thesurvival outcome of this procedurewith that of conventional axillary noderesection have not been completed.”Specifically, they are awaiting the resultsof the American College of SurgeonsOncology Group (ACOSOG)Z0011 trial and the National SurgicalAdjuvant Breast and Bowel Project(NSABP) B-32 trial before they makea determination about the “safety” ofthis procedure.[1] This is a commonmisconception, because these studieswill not prove whether sentinel nodebiopsy results in equivalent survivalcompared to standard level I/II axillarydissection in patients with clinicallynode-negative breast cancer. Thetime has come for clear thinking aboutthe design of these trials and precisereasoning about the interpretation oftheir ultimate results.
Krag and Harlow believe thatsentinel lymph node biopsyfor breast cancer is still an experimentalprocedure because "longtermrandomized trials comparing thesurvival outcome of this procedurewith that of conventional axillary noderesection have not been completed."Specifically, they are awaiting the resultsof the American College of SurgeonsOncology Group (ACOSOG)Z0011 trial and the National SurgicalAdjuvant Breast and Bowel Project(NSABP) B-32 trial before they makea determination about the "safety" ofthis procedure.[1] This is a commonmisconception, because these studieswill not prove whether sentinel nodebiopsy results in equivalent survivalcompared to standard level I/II axillarydissection in patients with clinicallynode-negative breast cancer. Thetime has come for clear thinking aboutthe design of these trials and precisereasoning about the interpretation oftheir ultimate results.Contrasting Trial Designs
The ACOSOG Z0011 trial randomizespatients with positive sentinellymph nodes to completion axillarydissection or no axillary dissection. Itwill determine whether axillary dissectionin patients with positive nodesimproves survival-a long-standingand worthwhile question regarding thetherapeutic value of axillary dissection.It will not determine whether thesentinel node biopsy procedure resultsin reduced survival compared to standardaxillary dissection. Implicit in the design of this trial is the notion that patientswith negative sentinel nodes donot require any further axillary surgery.In contrast, the NSABP B-32 trialis based on the premise that axillarydissection in patients with negativenodes could improve survival. Kragand Harlow believe that the B-32 trialwill determine whether "sentinel nodesurgery results in a survival rate asgood as that of axillary node resection." However, the trial is designedas follows: Patients are randomized tosentinel node biopsy followed bycompletion axillary dissection (regardlessof the sentinel node result) vs sentinelnode biopsy with completionaxillary dissection only in patientswith positive sentinel nodes (Figure 1).It is important to note that NSABPB-32 patients with nodal metastasesare treated similarly in both groups-
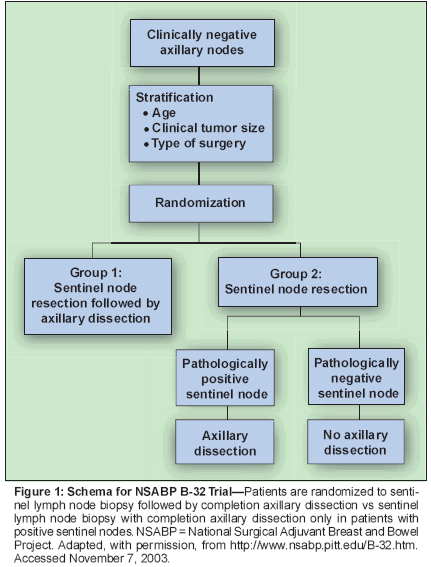
ie, they both undergo sentinel nodebiopsy and axillary dissection. Accordingly,a survival difference betweentreatment arms among node-positivepatients is impossible. Nonetheless,they are included in the randomization.Clearly then, any difference insurvival must occur among the nodenegativepatients.Axillary Dissection inNode-Negative Patients
One might reasonably ask, whywould it be beneficial to perform axillarydissection in patients with negativenodes? Can there be any advantageto removing normal lymph nodes,and is it really possible that sufficienthypothesis-generating evidence existsto support the contradictory notionsthat axillary dissection can be avoidedsafely in patients with positive nodes(ACOSOG Z0011) but improves survivalin patients with negative nodes(NSABP B-32)? Of course not. In fact,there are no convincing data to suggestthat removing normal lymphnodes will improve survival.[2] Kragand Harlow suggest that the 10-yearfollow-up data from the landmarkNSABP B-04 trial showed a 4% differencein survival favoring axillarydissection. Conveniently, they omit the25-year follow-up data, which demonstratedthat this supposed survivaladvantage completely disappears withlonger follow-up.[3]Based on a meta-analysis of six studies,including the 10-year follow-up datafrom B-04, it has been suggested thatthere is a survival advantage of as muchas 5.4% favoring axillary dissection.[4]This meta-analysis suffers from the significantlimitation that B-04 is the onlystudy that directly compared axillarydissection to no axillary dissection. Inall of the other studies, the control group(no axillary dissection) comprised ahodgepodge of radiation treatments,including radiation therapy to the axillary,supraclavicular, and, in all cases,internal mammary nodes.It is not difficult to believe that radiationtherapy, especially internalmammary treatment using techniquesfrom the 1950s and 1960s, couldresult in cardiac toxicity significantenough to negatively affect survival.[5-7] Keep in mind that thesestudies were all performed in an eraprior to modern systemic adjuvanttherapy, which likely would diminishany potential therapeutic benefit ofaxillary dissection.Investigational Ironies
I believe that axillary dissection isan excellent procedure for regionaldisease control, and I do not discountthe possibility that it imparts a smallsurvival advantage to patients withpositive nodes. But even if one believesthat axillary dissection improvessurvival, does anyone truly believe thatthere would be any survival advantageif node-positive patients were excluded?How ironic that the NSABP-which has for years taught us that axillarylymph node dissection is a stagingprocedure with no impact on survival-is now sponsoring a trial basedon the premise that axillary dissectionnot only improves survival, but improvessurvival in patients with negativelymph nodes.In truth, the only way there couldbe a legitimate survival difference betweentreatment arms in the NSABPB-32 study is for the sentinel nodefalse-negative rate to be so high that itaffects survival, but even that is impossible.Suppose that, of 100 patients,33 have positive axillary nodes. Evenwith a false-negative rate of 15% (theunacceptably high end of the reportedliterature), that would mean that 15%of 33 patients, or 5 of 100 patientswould be inaccurately staged as havingnegative axillary nodes. Even if weassume the 5.4% survival advantage foraxillary dissection from the meta-analysis,that would equate to 5.4% of 5 patients,or an absolute increase in survivalof 0.27% of our 100 patients. And evenif axillary dissection in patients withpositive nodes resulted in a survivaladvantage of as much as 25% (unlikely),this would only increase survival by1.25%. NSABP B-32 is designed todetect a 2% survival difference.Appropriate Trial Design
If one wanted to prove that sentinellymph node biopsy is equivalentto axillary node dissection in terms ofsurvival, the trial design is obvious:
Randomize patients to standard levelI/II axillary dissection vs sentinel nodebiopsy (with completion axillary dissectionfor patients with sentinel nodemetastases). In this way, the goldstandard procedure-axillary dissection-would be compared to the procedurethat seeks to replace it. Such astudy design would determine, for example,whether increased mortality, recurrence,or other unknown adverseoutcomes are related to the sentinel nodeprocedure (eg, caused by tumor manipulation,radioactive tracers, allergicreaction to blue dye, or other factorsbeyond our present comprehension).However, the safety of sentinel nodebiopsy cannot be addressed by a trial(ie, NSABP B-32), in which all patientsundergo sentinel node biopsy. Itis equally likely that sentinel node biopsycould improve survival by detectionof early nodal metastases thatwould otherwise be missed by routinepathologic analysis of the axillary dissectionspecimen. This more appropriaterandomized study design has beenemployed in the ongoing AxillaryLymphatic Mapping Against NodalAxillary Clearance (ALMANAC)trial.[8] However, the primary endpoints of that study are axillary morbidity,health economics, and qualityof life. A secondary goal is to evaluateaxillary recurrence. Specifically,this study is not designed or poweredto evaluate overall survival.Impact of NSABP B-32
When the predictably negative resultsof NSABP B-32 are released,many who have not carefully consideredthese issues will proclaim that thisstudy provides the final proof that sentinelnode biopsy is safe and can beaccepted as a standard procedure foraxillary nodal staging. The study willprovide much important informationabout the prognostic significance ofnodal micrometastases and many otherfactors, and has been an excellentmechanism for surgical training in thecontext of a cooperative group trial.Contrary to what Drs. Krag andHarlow profess that NSABP B-32 willshow, however, it will not tell uswhether sentinel node biopsy reduces overall survival compared to standardaxillary dissection. It will prove onlywhat we already know-that there isno advantage to removing normallymph nodes.Conclusions
After all, sentinel node biopsy is notdesigned to be a therapeutic procedureor to improve survival-it is a diagnosticstaging test to determine the statusof the axillary nodes. It is a lessinvasive alternative to axillary dissection.As with any other diagnostic test,the validity of sentinel node biopsy isnot accomplished by randomization,but by performing nonrandomizedvalidation studies to determine the sensitivity,specificity, positive- and negative-predictive values, overall accuracy,and false-negative rate.[9] Theresults of such studies have been reportedfor thousands of patientsinternationally.[10]Once surgeons have establishedthat the procedure can be performed accurately, there is no reason not to offersentinel node biopsy to women whoare informed that they are trading asmall chance of a false-negative resultfor a less invasive test. That is why sentinelnode biopsy is no longer an experimentalprocedure in the hands ofexperienced surgeons and is currentlypracticed as a standard of care in thousandsof centers around the world.[11]
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Krag D, Ashikaga T: The design of trialscomparing SLN surgery and axillary resection.N Engl J Med 349:603-605, 2003.
2.
McMasters KM: The eternally enigmaticaxilla: Further controversy about axillarylymph nodes in breast cancer. Ann Surg Oncol.In press.
3.
Fisher B, Jeong JH, Anderson S, et al:Twenty-five-year follow-up of a randomizedtrial comparing radical mastectomy, total mastectomy,and total mastectomy followed by irradiation.N Engl J Med 347:567-575, 2002.
4.
Orr RK: The impact of prophylactic axillarynode dissection on breast cancer survival-a Bayesian meta-analysis. Ann Surg Oncol6:109-116, 1999.
5.
Freedman GM, Fowble BL, Nicolaou N,et al: Should internal mammary lymph nodesin breast cancer be a target for the radiationoncologist? Int J Radiat Oncol Biol Phys46:805-814, 2000.
6.
Cuzick J, Stewart H, Rutqvist L, et al:Cause-specific mortality in long-term survivorsof breast cancer who participated in trials ofradiotherapy. J Clin Oncol 12:447-453, 1994.
7.
Early Breast Cancer Trialists’ CollaborativeGroup: Effects of radiotherapy and surgeryin early breast cancer. N Engl J Med 333:1444-1455, 1995.
8.
Clarke D, Khonji NI, Mansel RE: Sentinelnode biopsy in breast cancer: ALMANACTrial. World J Surg 25:819-822, 2001.
9.
McMasters KM, Giuliano AE, Ross MI,et al: Sentinel lymph node biopsy for breastcancer-not yet the standard of care. N Engl JMed 339:990-995, 1998.
10.
Chao C, McMasters K: The current statusof sentinel lymph node biopsy for breastcancer. Adv Surg 36:167-192, 2002.
11.
Schwartz GF, Giuliano AE, Veronesi U,et al: Proceedings of the consensus conferenceon the role of sentinel lymph node biopsy incarcinoma of the breast, April 19-22, 2001,Philadelphia, Pennsylvania. Cancer 94:2542-2551, 2002.