Paclitaxel and Gemcitabine as Salvage Treatment in Metastatic Breast Cancer
Both paclitaxel and gemcitabine (Gemzar) have shown activity andmanageable toxicity when used as single agents in heavily pretreatedpatients with metastatic breast cancer. This phase II study evaluatedtheir use in combination for metastatic breast cancer patients whosedisease recurred or progressed following treatment with anthracyclinecontainingregimens.
ABSTRACT: Both paclitaxel and gemcitabine (Gemzar) have shown activity andmanageable toxicity when used as single agents in heavily pretreatedpatients with metastatic breast cancer. This phase II study evaluatedtheir use in combination for metastatic breast cancer patients whosedisease recurred or progressed following treatment with anthracyclinecontainingregimens. Twenty-nine patients ranging from 32 to 68 yearsof age received paclitaxel at 175 mg/m² IV over 3 hours on day 1 andgemcitabine at 1,000 mg/m² IV on days 1, 8, and 15 every 28 days.Because of unacceptable thrombocytopenia in the first five patients,the gemcitabine schedule was changed to days 1 and 8 of a 21-daycycle for the remainder of the study. All 29 patients were evaluable forresponse and toxicity. Seventeen patients (59%) were considered trulyanthracycline- or anthracenedione-refractory. A total of 137 cycles(median: 4 per patient) were administered. The regimen was well tolerated.Grade 3/4 thrombocytopenia was observed in 5 (18.5%) of thefirst 27 cycles and in 6 (5.4%) of the 110 cycles following dosage reduction(P = .04). Five patients had grade 1 and two patients had grade 3neuropathy. Eight patients had grade 3 neutropenia, two had grade 4neutropenia with fever at the higher dosage, and eight had grade 1/2myalgia and fatigue. Five patients (17%) had a complete response and11 (38%) a partial response, yielding an objective response rate of 55%(95% confidence interval = 36%â73%). Six patients (20.7%) had stabledisease. Median response duration was 8 months (range: 4â26 months),and median overall survival was 12 months (range: 4â48+ months).Survival at 1, 2, 3, and 4 years was 45%, 30%, 20%, and 10%, respectively.The combination of paclitaxel on day 1 with gemcitabine on days1 and 8 of a 21-day cycle appears to have promising activity in heavilypretreated patients with metastatic breast cancer. Phase III trials comparingthis promising doublet to paclitaxel monotherapy and to otherchemotherapeutic strategies for advanced breast cancer will clarify therole of this regimen.Treatment of refractory metastaticbreast cancer remains amajor challenge to the medicaloncologist. Second-line treatment afterfailure of initial chemotherapy isprimarily palliative.[1] Although objectiveresponses can be produced withvarious drug combinations in about20% to 40% of cases, nearly all patientstend to show rapid tumor progression.[2,3] Therefore, efforts at improvingthe outcome of patients withthis disease are of major interest.Previous TrialsPaclitaxel as Monotherapy
Paclitaxel, a novel antimicrotubuleagent derived from the bark of thewestern yew Taxus brevifolia, is oneof the most active anticancer drugsintroduced into the clinic during recentyears.[4] Unlike other microtubulepoisons in use, such as vincristineand vinblastine, paclitaxel promotesthe formation of tubulin dimersand stabilizes microtubules against depolymerization,resulting in growthinhibition and loss of cell viability.[5,6]Early phase II trials of paclitaxelrevealed significant antitumor activity in various types of solid tumors, includingovarian,[7] non-small-celllung,[8] and head and neck[9] carcinomas.In previously treated metastaticbreast cancer patients, paclitaxelgiven at doses ranging from 135 to 250mg/m2 has produced objective responsesin 30% to 60% of cases.[10]In a phase II trial performed atMemorial Sloan-Kettering CancerCenter, where 250 mg/m2 of paclitaxelwas given as initial therapy for advanceddisease as a continuous IV infusionevery 21 days with hematologicsupport (recombinant human granulocytecolony-stimulating factor), objectiveresponses were documented in 16(62%) of 26 evaluable patients, includingthree (12%) complete responses.Although neutropenia was the doselimitingtoxicity, the incidence oflife-threatening infectious complicationsin that study was consideredacceptable.[11]These observations were confirmedin other phase II trials, where 175 to250 mg/m2 of the drug was given as a3-hour IV infusion.[12,13] With theconfirmation of initial reports of theantitumor activity of single-agentpaclitaxel in metastatic breast cancerpatients who have undergone priorchemotherapy, interest in this agenthas increased substantially.[9-12]Nabholtz et al,[14] in a multicenterrandomized trial, compared two differentdoses of paclitaxel given as a3-hour infustion in patients with metastaticbreast cancer who had failed torespond to previous chemotherapy. Atotal of 471 patients were randomizedto receive intravenous paclitaxel at adose of 175 or 135 mg/m2 every 3weeks. Better treatment results wereachieved with high-dose vs low-dosepaclitaxel: overall response rate, 29%vs 22% (P = .108); complete responserate, 5% vs 2% (P = .088); mediantime to disease progression, 4.2 vs 3.0months (P = .027); and median survivaltime, 11.7 vs 10.5 months (P =.321). Patients previously exposed orresistant to anthracyclines were aslikely to respond as those without suchprior exposure.Treatment was well tolerated, asdocumented by the number of administeredtreatment courses (median, 6[range:1-17] vs 5 [range:1-18]), thelow frequency of dose reductions(14% vs 7%, P = .024), and the smallnumber of patients (n = 9 [4%] vs n =5 [2%]) who required treatment discontinuationfor adverse reactions.The incidence and severity of neutropeniaand peripheral neuropathy weredose-related. After quality-of-life adjustedtime-to-progression analysis,the 175 mg/m2 arm retained its advantageover the 135 mg/m2 arm.
Follow-up studies have not onlyconfirmed the partial lack of clinicalcross-resistance between paclitaxeland anthracyclines, but have also revealedits significant single-agent activityin nonpretreated patients.[10,11]Indeed, paclitaxel can be consideredat least as active as doxorubicin whenused as a single agent in phase II trialsthat have been conducted in patientswith metastatic breast cancer. For thatreason, it has been progressively incorporatedinto experimental combinationregimens for both first-line and salvagetreatment of this disease.[15-19]Gemcitabine as Monotherapy
Gemcitabine (Gemzar) is a novelnucleoside analog of deoxycytidinewith a broad range of activity againstvarious tumors and an especially favorabletoxicity profile, including mildmyelosuppression and minimalnonhematologic toxicity.[20] Several important phase II studies of singleagentgemcitabine as first- or secondlinechemotherapy have been conductedin patients with metastaticbreast cancer.In a US study,[21] gemcitabine at1,200 mg/m2, given on days 1, 8, and15 as first-line palliative therapy,achieved an overall response rate of46% (2 complete responses and 10partial responses among 26 evaluablepatients). The same gemcitabine regimenadministered to patients who receiveda prior anthracycline-basedregimen resulted in a remission rateof 30% (2 complete and 6 partial responsesamong 27 evaluable patients).[22]In a study known as the "European"study,[23] gemcitabine was given asfirst- or second-line therapy at a meandose of 725 mg/m2 once weekly for 3weeks, followed by a rest period of 4weeks. Although this dose was relativelylow, patients had a remissionrate of 25% (3 complete and 7 partialresponses among 40 evaluable patients).A study was initiated that useda similar regimen but a highergemcitabine dose (1,000 mg/m2) ondays 1, 8, and 15 of a 4-weekcycle.[24] Of the 40 patients evaluable for response, there were three completeand seven partial responders, foran overall response rate of 25%.Twenty-six of these patients had receivedprior chemotherapy (includingseven in the adjuvant setting). All patientshad stage IV disease. Mediansurvival reported in these two studieswas 11.5 months.In a recent study that used the samegemcitabine regimen, no complete responseswere observed, but there weresix partial responses, for an overallresponse rate of 14.3% among 42evaluable patients.[25] Median survivalfor all 42 patients was 15.2months. Patients received up to oneprior chemotherapy regimen in theadjuvant setting. The majority of patients(67%) had visceral disease atstudy entry.In these phase II studies, hematologicand nonhematologic toxicitieswith single-agent gemcitabine treatmentwere mild, with neutropenia being themost clinically relevant untoward event.Treatment delays or withdrawals fromtreatment were infrequent.Considering the single-agent activityof both paclitaxel and gemcitabinein metastatic breast cancer patients,their different mechanisms of action,and their distinct nonhematologic toxicityprofile, we designed this phaseII trial in which a combination of thetwo drugs was evaluated as salvagetherapy in patients failing first- or second-line chemotherapy regimens.
Patients and MethodsEligibility Criteria
Eligible patients had histologicallyconfirmed metastatic breast cancerwith bidimensionally measurable disease.All had already received first- orsecond-line therapy with an anthracycline-containing regimen, but hadno prior exposure to paclitaxel or gemcitabine.Other eligibility criteria includedan Eastern Cooperative OncologyGroup (ECOG) performance statusof 0 to 2, age > 70 years with ananticipated life expectancy > 12 weeks,adequate renal and hepatic function, noactive infection, no central nervous systeminvolvement or evidence of carcinomatousmeningitis, white blood cellcount ≥ 4 * 109/L, absolute neutrophilcount ≥ 2 * 109/L, hemoglobin level≥ 10 g/dL, platelet count ≥ 130 * 109/L,and signed written informed consent.Exclusion criteria included a historyof prior malignancy other thannonmelanoma skin cancer or cervicalcarcinoma in situ, significant cardiacdisease, and a history of or existingperipheral neuropathy. Prior radiationcompleted at least 4 weeks from the dateof study registration was permitted, aslong as it encompassed less than 30%of the total marrow-bearing skeleton.Any hormonal therapy was discontinuedat least 3 weeks before study entry.Treatment Plan
Twelve hours before administrationof paclitaxel, all patients were givenoral 8 mg of dexamethasone. This dosewas repeated IV 15 minutes beforepaclitaxel was started. In addition, dimenhydrinateat 100 mg IV, ranitidineat 50 mg IV, and promethazine at50 mg intramuscularly were given 30minutes prior to paclitaxel administration.Treatment on day 1 consisted ofpaclitaxel at 175 mg/m2 IV (diluted in500 mL of 0.9% normal saline andinfused over a period of 3 hours), followedby gemcitabine at 1,000 mg/m2IV (diluted in 250 mL of 0.9% normalsaline). On day 8, gemcitabinealone was given at the same dose(schedule G-1,8). Cycles were repeatedevery 21 days on an outpatientbasis for a maximum of eight cycles.In the first five patients, gemcitabinewas given at the same dose on days 1,8, and 15 every 28 days (schedule G-1,8,15). However, this produced anunacceptable level of thrombocytopenia,and the regimen was modified toG-1,8 every 21 days.Cycles were repeated if the neutrophilcount exceeded 1 * 109/L andplatelet count exceeded 120 * 109/L.If either of these hematologic parameterswere not within their respectiverange on the scheduled treatment day,therapy was delayed for a week. If aftera delay of 2 weeks these valueswere still not in the appropriate range,the patient was removed from thestudy. The doses of paclitaxel andgemcitabine were reduced by 50% inthe treatment cycle if febrile neutropeniaoccurred or if a neutrophil countnadir < 0.5 * 109/L or platelet countnadir < 50 * 109/L was documented.Patients with progressive disease afterthe second cycle and those whosedisease stabilized or progressed afterthe fourth cycle of chemotherapy werealso removed from the study.Pretreatment Evaluationand Follow-up Studies
Before protocol enrollment, all patientsunderwent a complete historyand physical examination. Laboratoryevaluation included a complete bloodcount (CBC) with differential andplatelet count, a urinalysis and biochemicalprofile, determination of serumCA 15-3 and lactate dehydrogenaselevels, a baseline electrocardiogram,assessment of ECOG performancestatus, chest x-ray, and ultrasoundexamination and computed tomographyof the abdomen and suspiciousareas, including a bone scan. ACBC was repeated on day 8 and day15, initially, of each cycle. Physicalfindings, ECOG performance status,CBC with differential and platelets, biochemicalprofile, and serum CA 15-3were reevaluated after each cycle.Complete tumor measurements weredocumented at the end of the second cycle and again at the end of the study.Patients responding to therapy had torepeat the evaluation 4 weeks laterusing the same methods for responseconfirmation.
Response Criteria
All eligible patients were consideredfor response analysis. Lesions(eg, metastatic pulmonary nodules,lymph nodes, subcutaneous masses,and hepatic metastases) had to be measurablein two dimensions with rulersor calipers, and their surface area wasdetermined by multiplying the longestdiameter by the greatest perpendiculardiameter. Bone lesions onlywere not considered measurable disease.For multiple lesions, total tumorsize was defined as the sum of theproducts of the largest perpendiculardiameters of each lesion.[26]The definition of response wasbased on World Health Organization(WHO) recommendations.[27] Completeresponse meant the complete disappearanceof all known disease determinedby two observations made noless than 4 weeks apart, without theappearance of a new lesion. Partialresponse was documented by a decreaseof at least 50% (for bidimensionallymeasurable lesions) of thesum of the products of the two perpendiculardiameters of all measuredlesions and no new lesion or progressionof any lesion, based upon twoobservations made no less than 4weeks apart. No change was considereda decrease of > 50%, or < 25%increase in the sum of the products ofthe two perpendicular diameters of allmeasured lesions and no new lesions.Progressive disease meant an increaseof at least 25% in the size of at least one measurable lesion or the appearance ofa new lesion. The occurrence of pleuraleffusion or ascites was also consideredprogressive disease if documented bypositive cytology. Pathologic fracturewas not automatically considered evidenceof disease progression.Evaluation of Adverse Events
Any patient who had received atleast one course of paclitaxel/gemcitabine was evaluable for safetyand toxicity. The type and severity ofthese toxicities was determined usingWHO criteria.Statistical Considerations
The two-stage phase II design describedby Gehan was applied to thisstudy.[28] The 95% confidence intervals(CI) for response rates were calculatedusing the binomial theorem.Survival and response duration werecalculated using the Kaplan-Meiermethod, using a microcomputer-assistedprogram.[29,30]ResultsPatient Characteristics
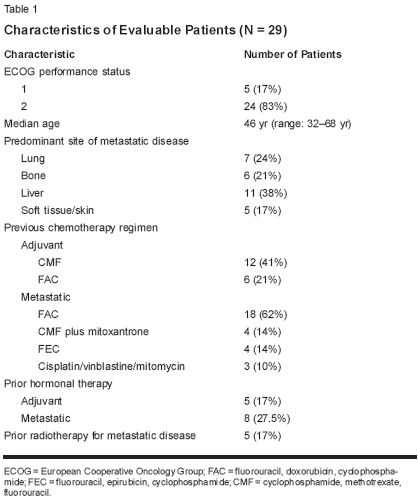
Twenty-nine patients with a medianage of 46 years (range: 32-68 years)were enrolled. All were consideredeligible for evaluation of response andtoxicity. Patient characteristics aresummarized in Table 1. Previous chemotherapyincluded (1) fluorouracil(5-FU), doxorubicin (Adriamycin),and cyclophosphamide (Cytoxan,Neosar), known as FAC (14 cases offirst-line therapy and four cases of second-line therapy after failure of cyclophosphamide,methotrexate, and5-FU [CMF]); (2) 5-FU, epirubicin(Ellence), and cyclophosphamide, or FEC (four cases of first-line therapy);(3) mitoxantrone (Novantrone) plusCMF (four cases of first-line therapy);and (4) cisplatin, vinblastine, and mitomycin(Mutamycin) (three cases ofsecond-line therapy after failure ofFAC). All but four patients had a tumorresponse with prior chemotherapy.Seventeen (59%) patientswere considered truly anthracyclineoranthracenedione-refractory (ie, theyhad progressed during the use of regimenscontaining these agents). Themedian follow-up was 22 months.Safety and Toxicity
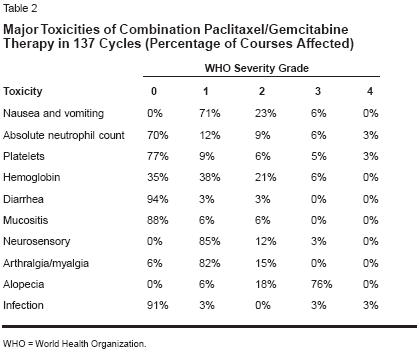
A total of 137 cycles (median: 4 perpatient) were administered. Treatmentdelays occurred in 13 cycles (9.5%),and dose reductions were needed in17 (12.4%) cycles. Cycle delays occurredin eight cycles (5.8%) due tothrombocytopenia and in four cycles(2.9%) due to neutropenia. Dose reductionsdue to myelotoxicity occurredin nine cycles (6.6%). No hypersensitivityreactions were seen.The regimen was well tolerated(Table 2). Nausea/vomiting (grade 1),
The regimen was well tolerated(Table 2). Nausea/vomiting (grade 1),alopecia (grades 2/3), and neutropenia(grade 1) were seen in most patients.Five patients had grade 1 andtwo patients had grade 3 neuropathy.Grade 3/4 thrombocytopenia was observedin five (18.5%) of the first 27cycles (schedule G-1,8,15), althoughno bleeding was observed, and in six(5.4%) of the 110 subsequent cycles(schedule G-1,8). The difference infrequency of grade 3/4 neutropeniabetween the two dosing schedules wassignificant (P = .04, Fisher's exacttest). Eight patients had grade 3 neutropenia(5 with schedule G-1,8,15).In addition, grade 4 neutropenia wasassociated with fever in two cases andin four cycles (schedule G-1,8,15).Three patients received blood transfusionsdue to anemia. Grade 1/2 myalgiaand fatigue were also reported ineight patients. One patient developeda reversible bradycardia duringpaclitaxel infusion. No death due totoxicity occurred.Responses and Survival
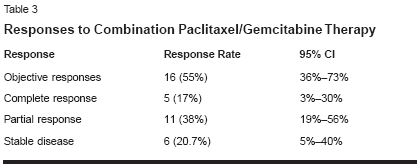
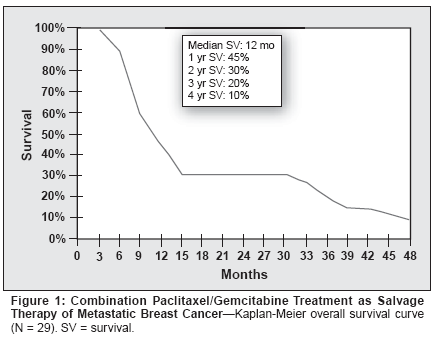
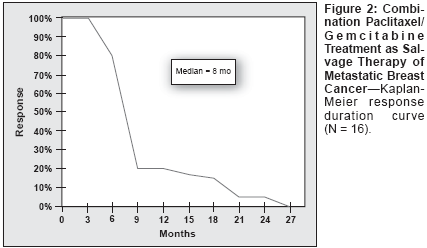
There were 16 (55%) objective responses(95% CI = 36%-73%), includingfive (17%) complete responses(95% CI = 3%-30%) and 11 (38%)partial responses (95% CI = 19%-56%). Six (20.7%) patients attaineddisease stabilization (Table 3). Medianresponse duration was 8 months(range: 4-26 months), and medianoverall survival was 12 months(range: 4-48+ months). Survival at 1,2, 3, and 4 years was 45%, 30%, 20%,and 10%, respectively. The overallsurvival curve is depicted in Figure 1and the response duration curve inFigure 2.Addition of Trastuzumab
Twenty-two patients were tested forHER2/neu overexpression after tumorprogression. In seven, HER2/neu was3+ positive by immunohistochemistryanalysis (HercepTest with monoclonalantibody 4D5). For these patients weadded trastuzumab (Herceptin) at 4mg/kg IV (loading dose), followed by2 mg/kg IV weekly until progressionto the same regimen of paclitaxel andgemcitabine for four additional cycles(postprotocol therapy, at the discretionof the treating oncologist). In three patients(42.9%) we observed a partial response, with a median response durationof 5 months (range: 3-11+months).DiscussionOverall objective response rates ofmetastatic breast cancer to second- orthird-line chemotherapy have beenvery limited, regardless of the choiceof drug regimen.[1-3] The modestimpact of cytotoxic agents in this settingcan be explained, at least in part,by the high percentage of patients exhibitinga poor performance status atthe start of treatment, the presence ofbulky disease at visceral sites, limitedbone marrow reserve, and the presenceof highly drug-resistant tumorcells selected by prior exposure tochemotherapy.[1,31] Indeed, variousreviews on the effectiveness of combinationchemotherapy in this settingrevealed objective response rates below30%, and the responses were usuallypartial, of short duration, and atthe cost of significant toxicity to thepatient.[1-3,31]Our results confirm the significantantitumor activity of paclitaxel in combinationwith gemcitabine as secondorthird-line chemotherapy for patientswith metastatic breast cancer. Notably,this combination produced objectiveresponses in about half of the 29 patientstested and achieved a completeresponse in five (17%) of them. Basedon current literature, neither agentgiven alone would have been expectedto produce such a substantial responserate. The median overall survival of 12months, as well as the 1-, 2-, and 3-year survival rates of 45%, 30%, and20%, respectively, are remarkable andunusual for this subgroup of patients.It is interesting to note that for sevenHER2/neu 3+ positive patients,trastuzumab was added to the sameregimen of paclitaxel and gemcitabinefor four more cycles, after progressionon the protocol. In three (42.9%) ofthe seven patients we observed a partialresponse, with a median responseduration of 5 months (range: 3-11+months), which raises the question ofwhether anti-HER2/neu therapiescould restore gemcitabine or paclitaxeltumor cell sensitivity.Despite the theoretical backgroundfor possible synergy betweengemcitabine and paclitaxel due to theirdifferent cellular mechanisms of action,a recent report using in vitro concurrentor sequential treatment of severalcell lines with gemcitabine andpaclitaxel showed an antagonistic effect.[32] Although our study was notdesigned to test antagonism or in vivosynergy between paclitaxel andgemcitabine, our preliminary resultsdo not suggest antagonism in the clinicalsetting. Further study of the combinationmay be necessary to addressthose specific issues.Importantly, the degree of efficacyobserved in this study did not occur atthe cost of unacceptable toxicity. Onthe contrary, toxicity appeared to bepredictable and manageable. Omissionof day-15 gemcitabine significantlyimproved the frequency of grade 3/4thrombocytopenia. In addition, therewere no further reports of fever andneutropenia when gemcitabine wasadministered only on days 1 and 8.[33]This suggests that the best schedule forthis combination should be G-1,8; ie,with paclitaxel given on day 1 andgemcitabine given on days 1 and 8 ofa 21-day cycle.Our results suggest that the combinationof paclitaxel and gemcitabineis very promising and may be an attractiveoption in the management ofpatients with metastatic breast cancer.Due to its significant efficacy, as wellas manageable toxicity, this regimendeserves further testing in both minimallyand heavily pretreated patientswith advanced disease. The additionof trastuzumab to this regimen shouldalso be explored.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Henderson CI: Chemotherapy of breastcancer: A general overview. Cancer 51:2553-2559, 1983.
2.
Hayes DF, Henderson IC, Shapiro CL:Treatment of metastatic breast cancer: Presentand future prospects. Semin Oncol 22(suppl5):5-21, 1995.
3.
Norton L: Salvage chemotherapy of breastcancer. Semin Oncol 21:19-24, 1994.
4.
Vogel CL: Current status of salvage chemotherapyfor refractory advanced breast cancer.Oncology 10(suppl 6):7-15, 1996.
5.
Kingston DGI, Samaranayake G, Ivey CA:The chemistry of taxol, a clinically useful anticanceragent. J Nat Prod 53:1-12, 1990.
6.
Wani MC, Taylor HL, Wall ME, et al:Plant antitumor agents VI: The isolation andstructure of taxol, a novel antileukemic andantitumor agent from taxus brevifolia. J AmChem Soc 893:2325-2327, 1971.
7.
McGuire WP, Rowinsky EK, RosensheinNB, et al: Taxol: A unique antineoplastic agentwith significant activity in advanced ovarianepithelial neoplasms. Ann Int Med 11:273-279,1989.
8.
Chang A, Kim K, Glick J, et al: Phase IIstudy of taxol in patients with stage IVnonsmall cell lung cancer: The Eastern CooperativeStudy Group study results (abstract).Proc Am Soc Clin Oncol 11:294, 1992.
9.
Forastiere AA, Adams G, Neuberg D, etal: Phase II trial of taxol in head and neck cancer:An Eastern Cooperative Study Group study(abstract). Proc Am Soc Clin Oncol 12:329,1993.
10.
Holmes FA, Walters RS, Theriault RL,et al: Phase II trial of Taxol, an active drug inthe treatment of metastatic breast cancer. J NatlCancer Inst 83:1797-805, 1991.
11.
Seidman AD, Tiersten A, Hudis C, et al:Phase II trial of paclitaxel by 3-hour infusionas initial and salvage chemotherapy for metastaticbreast cancer. J Clin Oncol 13:2575-2581, 1995.
12.
Reichman BS, Seidman AD, Crown JPA,et al: Paclitaxel and recombinant human granulocytecolony-stimulating factor as initial chemotherapyfor metastatic breast cancer. J ClinOncol 11:1943-1951, 1993.
13.
Holmes FA, Valero V, Walters RS, et al:The M.D. Anderson Cancer Center experiencewith taxol in metastatic breast cancer. J NatlCancer Inst Monogr 15:161-169, 1993.
14.
Nabholtz JM, Gelmon K, Bontenbal M,et al: Multicenter, randomized comparativestudy of two doses of paclitaxel in patients withmetastatic breast cancer. J Clin Oncol 14:1858-1867, 1996.
15.
Gianni L, Munzone E, Capri G, et al:Paclitaxel by 3-hour infusion in combinationwith bolus doxorubicin in women with untreatedmetastatic breast cancer. High antitumorefficacy and cardiac effects in a dose-findingand sequence-finding study. J Clin Oncol13:2688-2699, 1955.
16.
Schwartsmann G, Menke CH, et al: PhaseII trial of taxol, plus doxorubicin plus G-CSF inpatients with metastastic breast cancer (abstract).Proc Am Soc Clin Oncol 15:126, 1996.
17.
Klaassen U, Wilke H, Philippou Pari C,et al: Phase I/II study with paclitaxel in combinationwith weekly high-dose 5-FU/folonicacid in the treatment of metastatic breast cancer(abstract 186). Proc Am Soc Clin Oncol15:122, 1995.
18.
Murad AM, Guimarães RC, AmorimWC, et al: Phase II trial of paclitaxel andifosfamide as a salvage treatment in metastaticbreast cancer. Breast Cancer Res Treat 45:47-53, 1997.
19.
Rosenthal CJ, Ibrahim A, Rambhia H,et al: Paclitaxel (Taxol) mitoxantrone salvagetherapy in metastatic carcinoma of the breast:Phase I/II study (abstract). Proc Am Soc ClinOncol 13:92, 1994.
20.
Plunkett W, Huang P, Xu YZ, et al:Gemcitabine: Metabolism, mechanisms of ac tion, and self-potentiation. Semin Oncol 22:3-10, 1995.
21.
Blackstein M, Vogel CL, Ambinder R,et al: Phase II study of gemcitabine in patientswith metastatic breast cancer (abstract 135).Proc Am Soc Clin Oncol 15:117, 1996.
22.
Speilmann M, Pouillard P, Espie M, etal: Activity of gemcitabine in metastatic breastcancer (MBC) in patients previously treatedwith anthracycline-containing regimens (abstract143). Breast Cancer Res Treat 41:236,1996.
23.
Carmichael J, Possinger K, Phillip P, etal: Advanced breast cancer: A phase II trial withgemcitabine. J Clin Oncol 13:2731-2736, 1995.
24.
Carmichael J, Walling J: Phase II activityof gemcitabine in advanced breast cancer.Semin Oncol 23(suppl 10):77-81, 1996
25.
Possinger K, Kaufmann M, Coleman R,et al: Phase II study of gemcitabine as firstlinechemotherapy in patients with advancedor metastatic breast cancer. Anticancer Drugs10(2):155-162, 1999.
26.
Carmichael J, Walling J: Advanced breastcancer: Investigational role of gemcitabine. EurJ Cancer 33(suppl 1):S27-30, 1997.
27.
WHO: Handbook for Reporting Resultsof Cancer Treatment. WHO Offset PublicationNo 48, World Health Organization, Geneva,Switzerland, 1979.
28.
Gehan EA: The determination of thenumber of patients required in a follow up trialof a new chemotherapy agent. J Chronic Dis13:346-353, 1961.
29.
Kaplan EL, Meier P: Nonparametric estimationfrom incomplete observations. J AmStat Assoc 53:457-81, 1958.
30.
Campos-Filho N, Franco ELF: Microcomputer-assisted univariate survival dataanalysis using Kaplan-Meier life table estimators.Comp Meth Prog Biomed 27:223-8, 1988.
31.
Norton L, Simon R: The Norton-Simonhypothesis revisited. Cancer Treat Rep 70:163-169, 1986.
32.
Theodossiou C, Cook JA, Fisher J, et al:Interaction of gemcitabine with paclitaxel andcisplatin in human tumor cell lines. Int J Oncol12:825-832, 1998.
33.
Murad AM, Guimaraes RC, Aragao BC,et al: Phase II trial of the use of paclitaxel andgemcitabine as a salvage treatment in metastaticbreast cancer (abstract). Am J Clin Oncol24:264-268, 2001.