Cutaneous Side Effects of Multikinase Inhibitors Used in Renal Cell Cancer
Paralleling the increasing use of multikinase inhibitors in the field of cancer therapy, patients and clinicians are confronted with frequently occurring cutaneous side effects associated with the use of these new drugs. Two such targeted agents, sunitinib (Sutent) and sorafenib (Nexavar), were recently approved by the US Food and Drug Administration to treat patients with metastatic renal cell cancer (RCC).
Click here to earn Continuing Medical Education Credit
Abstract
Paralleling the increasing use of multikinase inhibitors in the field of cancer therapy, patients and clinicians are confronted with frequently occurring cutaneous side effects associated with the use of these new drugs. Two such targeted agents, sunitinib (Sutent) and sorafenib (Nexavar), were recently approved by the US Food and Drug Administration to treat patients with metastatic renal cell cancer (RCC). Cutaneous adverse effects associated with these drugs are sometimes distressing, partly because of their chronicity given the long-term duration of treatments, and can result in treatment interruption. Active collaboration between dermatologists and medical oncologists or urologists is urgently needed to characterize and treat these dermatologic side effects.
Introduction
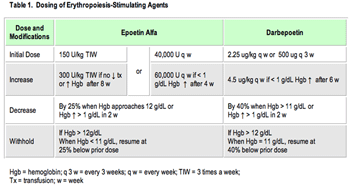
Sorafenib blocks Raf gene products (serine-threonine kinases), including mutated B-Raf, as well as platelet-derived growth factor-beta (PDGFR-β), FLT3, and vascular endothelial growth factor receptor-2 and -3 (VEGFR-2 and -3). Sunitinib blocks VEGFR-1, -2, and -3, PDGFR-α and -β, Ret, Kit, and FLT3. Both drugs were recently approved by the US Food and Drug Administration (FDA) to treat patients with metastatic RCC,1,2 and sunitinib has also been approved for patients with imatinib (Gleevec)-resistant gastrointestinal stromal tumors.3 Furthermore, both drugs are currently being tested in various tumors (such as sorafenib for hepatocarcinoma) as single agents and in combination with chemotherapy, with promising preliminary results in several tumors. Numerous cutaneous side effects have been observed with these two molecules.4 Some side effects are common to the two drugs, such as hand-foot skin reaction (HFSR), xerosis, and subungual splinter hemorrhages (SSH), whereas others, like hair modifications, keratotic papules, and facial erythema, seem to be associated with the use of one or another of these targeted agents. Table 1 shows the association between the targeted receptors and the skin symptoms observed.
Click to enlarge
Hand-Foot Skin Reaction
HFSR occurs in 30%−60% of patients receiving sorafenib and 15%−20% of patients treated with sunitinib,1 often appearing after 2 to 4 weeks of treatment. It seems to be dose dependent and rapidly disappears after treatment discontinuation.
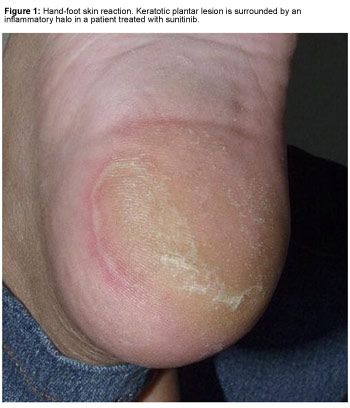
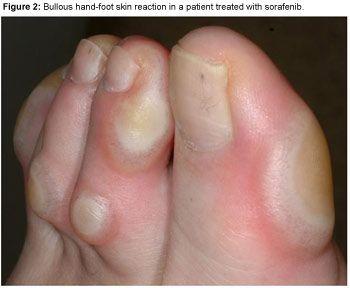
HFSR usually presents as painful symmetric erythematous and edematous areas on the palms and soles, often preceded or accompanied by paresthesia (tingling sensations and intolerance to contact with hot objects); these sensations seem to be aggravated by a warm environment. HFSR can also affect the lateral sides of fingers or the periungual zones. Hyperkeratosis and desquamation are often associated. Painful hyperkeratotic areas on pressure points, surrounded by rings of erythematous and edematous lesions, are classically observed (Figure 1), and painful bullous lesions may be seen (Figure 2). Sole involvement can result in walking impairment. Preexistent sole hyperkeratosis seems to confer a predisposition to painful sole involvement and functional consequences. Although this syndrome can sometimes clinically resemble the more classic chemotherapy-induced acral erythema (AE), kinase inhibitor-induced HFSR has more localized and hyperkeratotic lesions, distinct from classic AE.
Click to enlarge
Click to enlarge
Histologic examination of HFSR shows epidermal changes suggestive of keratinocyte differentiation modifications. The granular layer is thinner or absent in several specimens, and some areas of parakeratosis are seen. Epidermal cells are swollen in the superficial stratum spinosum. Numerous mitoses are seen in the basal layers and also above the basal and suprabasal layers, where normally they should not be found physiologically. Dyskeratotic keratinocytes suggestive of apoptotic cells are observed.
As a preventive measure, patients with plantar hyperkeratosis are advised to have a pedicure prior to treatment. During treatment, however, shock absorbers can be used to relieve painful pressure points, and sandals seem to be helpful in some cases. Symptomatic treatment with over-the-counter emollient and keratolytic ointments (containing salicylic acid or urea) can be used. When grade 3 HFSR occurs (pain interfering with function), a dose reduction (to 50% of the dose) or treatment interruption for 1−2 weeks is recommended until symptoms return to grade 1. When treatment is resumed, the dose should be decreased by one level; the full dose should be returned after 3−4 weeks. As is the case with most of the other side effects occurring with kinase inhibitors, resuming treatment is not always associated with similar side effects, in our experience.
Subungual Splinter Hemorrhages
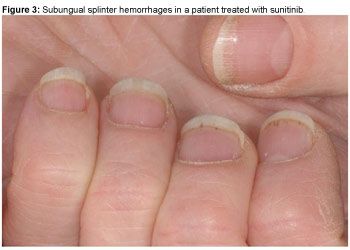
In our experience, multiple, painless distal SSH are frequently seen (in 40%−65% of patients) under fingernails (Figure 3) and less often under toenails of patients receiving multikinase inhibitors. First described in patients with infective endocarditis, SSH appear as linear black or red lines that actually look like wood splinters under the nails. They are formed and confined within the epidermis of the nailbed and consist of a mass of blood in a layer of squamous cells adhering to the undersurface of the nail. They are carried forward with nail growth and can be scraped away once they attain the free margin of the nail.
Click to enlarge
SSH are considered to be thrombotic or embolic in origin. Initially thought to be a typical sign of bacterial endocarditis, they have subsequently been reported to occur with various conditions,4 such as antiphospholipid syndrome, severe rheumatoid arthritis, thromboangiitis obliterans, and mitral stenosis; they also may occur at a high altitude or when arterial catheters are used. In addition, they may be seen in healthy patients, where they are more often confined to a single digit, and trauma seems to be the most common cause.5 Such traumatic SSH occur in the region where the nail plate separates from the nailbed, beneath which delicate, specialized, spiral capillaries are located and can easily rupture. Toxiderma is not usually an etiologic factor in SSH. SSH are rarely observed with imatinib and have not been reported with epidermal growth factor receptor (EGFR) inhibitors in the literature.
SSH may result from elective VEGFR blocking, which might prevent the physiologic repair of traumatized nailbed capillaries and facilitate the emergence of SSH.6 The hypothesis that nailbeds could offer a clinical monitoring window into the antiangiogenic effects of drugs that target VEGFR is being prospectively evaluated.
No therapeutic measure is needed for SSH, which are totally asymptomatic.
Xerosis
Skin dryness is a frequent complaint of patients treated with sorafenib and sunitinib. In our experience, skin dryness is reported in approximately 20%−30% of treated patients and is usually easy to treat symptomatically with skin emollient or body oil.
Facial and Scalp Erythematous Rash
A face and scalp rash is seen in more than 50% of patients after 1 or 2 weeks of treatment with sorafenib, in our experience. It appears as an erythematous and squamous rash involving the mediofacial area and the scalp skin, sparing the periocular areas. Reminiscent of classic moderate seborrheic dermatitis, it is frequently preceded by or associated with scalp dysesthesia. This rash frequently fades or disappears after several weeks of treatment and usually does not require treatment; however, if patients request therapy, a topical emollient, 2% ketoconazole, or level 2 or 3 topical steroids can be prescribed for a short time, depending on the severity of symptoms.
Hair Modifications
In our patients, the texture of scalp hair is frequently modified with sorafenib treatment: hair becomes more brittle, finer, and curly, sometimes resembling hair modification associated with the use of EGFR inhibitors. Some patients have reported darkening of hair color.
Click to enlarge
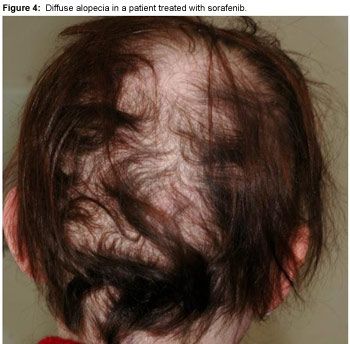
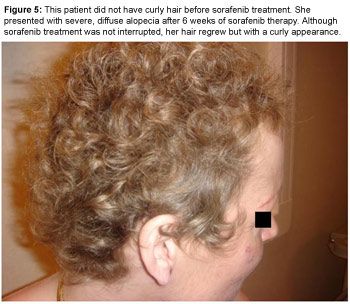
Alopecia may occur in some patients treated with sorafenib or sunitinib, but it is rarely severe (Figure 4). As opposed to EGFR inhibitor-induced alopecia, it is not located on the frontotemporal areas but rather as diffuse hair loss. Spontaneous resolution of alopecia usually occurs despite continued sorafenib treatment, and interestingly, regrowing hair is often curlier than it was prior to sorafenib treatment (Figure 5).
Click to enlarge
Sunitinib frequently gives rise to hair depigmentation after 5−6 weeks of treatment. It is reversible as early as 2−3 weeks after treatment discontinuation. Successive depigmented and normally pigmented bands that are correlated respectively with periods on and off treatment can be observed.7 Sunitinib-induced hair depigmentation is probably due to the blockade of Kit signalling, which modulates the expression of the melanogenesis enzymes via the microphthalmia transcription factor.
Keratotic Papules, Kystic Lesions, and Keratoacanthomas
The prevalence of this side effect is not yet known; however, we and others have observed several cases of multiple keratotic papules, cystic cutaneous lesions (from milia cysts to more inflammatory lesions), and keratoacanthomas in patients receiving sorafenib.8 Keratoacanthomas are autoinvolutive epidermal tumors with borderline frontiers with squamous cell carcinomas. One case of squamous cell carcinoma in a patient treated with sorafenib has been reported.9 Further studies are ongoing to understand the mechanisms leading to these cystic lesions and keratoacanthomas. Cystic noninflammatory lesions do not require treatment, unless they become inflammatory and/or infected. Topical antibiotic treatments, such as topical clindamycin, metronidazole, or erythomycin, are then indicated.
Lesions suggestive of keratoacanthomas should be surgically removed.
Yellowish Skin
Sunitinib is bright yellow, which may account for the yellowish skin coloration of patients treated with this drug. In our experience, yellowish skin is reported in about 30% of cases and is more visible in fair-skinned patients.
Periocular Edema
Periocular edema can be seen in sunitinib-treated patients. The preferential periocular location of the edema might be due to the high distension capacity of this skin area. Imatinib, which also targets PDGF and Kit receptors, is also known to be associated with edema, more frequently and severely than is sunitinib.10 Although the prevalent hypothesis explaining this symptom is the effect of PDGFR inhibition on interstitial fluid homeostasis, the exact mechanism leading to edema is not yet known. Moderate sunitinib periorbital edema does not usually require any treatment.
Conclusion
Inhibition of multikinase receptors results in a wide range of new and chronic cutaneous side effects. Some of these effects, like multiple subungual hemorrhages, facial erythema, moderate periocular edema, or hair modifications, are usually well tolerated. Nevertheless, physicians should be aware of them and should provide detailed information to their patients before introducing these drugs. Preventive measures can be taken for some of the skin effects, such as treatment of preexisting plantar hyperkeratosis to prevent HFSR.
Physicians also have to adapt the management of these cutaneous side effects in the context of long-term prescription of kinase inhibitors in renal cell cancer. Indeed, patients sometimes find it even more difficult to accept such chronic cutaneous side effects than acute and more severe effects. The study of these hitherto unknown side effects will provide useful information on skin, hair, and pigment cell physiology. Therefore, besides the mechanistic information generated as a result of their study, early recognition and symptomatic treatment of these associated cutaneous events should be critical aspects of the medical management of patients treated with kinase inhibitors. Synergistic collaboration between oncologists or urologists who treat patients with renal cell cancer and dermatologists is critical.
CME Post-Test and Evaluation
Continuing Medical Education Information
Cutaneous Side Effects of Multikinase Inhibitors Used in Renal Cell Cancer
Activity Release Date: May 1, 2007 Activity Expiration Date: May 1, 2008
About the Activity This activity is based on a brief article developed as part of the E-Update Series and posted on the Web. The series is geared to oncologists and addresses new treatments of cancer or modifications thereof.
This activity has been developed and approved under the direction of Beam Institute.
Activity Learning Objectives After reading this article, participants should be able to:
(a) Recognize the association between targeted receptors for metastatic renal cell cancer and skin symptoms.
(b) Identify an assortment of cutaneous side effects linked to the use of sorafenib and sunitinib.
(c) Discuss the role of preventive measures and treatment alternatives for these dermatologic adverse events.
(d) Understand the importance of synergistic collaboration between oncologists who treat patients with renal cell cancer and dermatologists.
Target Audience This activity targets physicians in the fields of oncology and hematology.
Accreditation This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Beam Institute and The Oncology Group. Beam Institute is accredited by the ACCME to provide continuing medical education for physicians
Continuing Education CreditAMA PRA Category 1 Credit™ The Beam Institute designates this educational activity for a maximum of 2 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Compliance Statement This activity is an independent educational activity under the direction of Beam Institute. The activity was planned and implemented in accordance with the Essential Areas and policies of the ACCME, the Ethical Opinions/Guidelines of the AMA, the FDA, the OIG, and the PhRMA Code on Interactions with Healthcare Professionals, thus assuring the highest degree of independence, fair balance, scientific rigor, and objectivity.
However, Beam Institute, the Grantor, and CMPMedica shall in no way be liable for the currency of information or for any errors, omissions, or inaccuracies in the activity. Discussions concerning drugs, dosages, and procedures may reflect the clinical experience of the author(s) or may be derived from the professional literature or other sources and may suggest uses that are investigational in nature and not approved labeling or indications. Activity participants are encouraged to refer to primary references or full prescribing information resources. The opinions and recommendations presented herein are those of the author(s) and do not necessarily reflect the views of the provider or producer.
Financial Disclosures Dr. Bukowski serves on the speakers' bureau for Bayer, Genentech, Pfizer, and Wyeth; is a consultant for Bayer, Genentech, Novartis, Pfizer, and Wyeth and receives research support from Pfizer and Wyeth. Dr. Motzer receives research support from Genentech, Pfizer, and Wyeth; and also serves on the speakers' bureau for Bayer. Dr. Escudier and Dr. Robert have no financial relationships with any manufacturers or providers.
Copyright Copyrights owned by Beam Institute, a division of CME LLC. Copyright 2007, CME LLC. All rights reserved.
Contact Information We would like to hear your comments regarding this or other activities provided by Beam Institute. In addition, suggestions for future activities are welcome. Contact us at:
Address: Director of Continuing Education Beam Institute 11 West 19th Street, 3rd Floor New York, NY 10011-4280
Phone: 888-618-5781 Fax: 212-600-3050 e-mail:beaminstitute@cmp.com
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-134.
2. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-124.
3. Joensuu H. Sunitinib for imatinib-resistant GIST. Lancet 2006;368:1303-1304.
4. Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 2005;6:491-500.
5. Young JB, Will EJ, Mulley GP. Splinter haemorrhages: facts and fiction. J R Coll Physicians Lond 1988;22:240-243.
6. Robert C, Faivre S, Raymond E, Armand JP, Escudier B. Subungual splinter hemorrhages: a clinical window to inhibition of vascular endothelial growth factor receptors? Ann Intern Med 2005;143:313-314.
7. Robert C, Spatz A, Faivre S, Armand JP, Raymond E. Tyrosine kinase inhibition and grey hair. Lancet 2003;361:1056.
8. Kong HH, Cowen EW, Azad NS, Dahut W, Gutierrez M, Turner ML. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol 2007;56:171-172.
9. Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol 2006;31:783-785.
10. Hensley ML, Ford JM. Imatinib treatment: specific issues related to safety, fertility, and pregnancy. Semin Hematol 2003;40(2 suppl 2):21-25.