Delaying Tamoxifen Until Chemotherapy Is Completed Improves Outcomes for Postmenopausal Breast Cancer Patients
CHICAGO-Giving tamoxifen concurrently with adjuvant chemotherapy halves the benefit of the chemohormonal combination compared to giving tamoxifen after completion of chemotherapy in postmenopausal patients with node-positive, hormone-receptor positive breast cancer (ASCO abstract 143). These initial results from North American Breast Intergroup Trial 0100 (INT 0100, SWOG 8814), presented by Kathy S. Albain, MD, are expected to establish a new standard of care for treating breast cancer in postmenopausal women.
CHICAGOGiving tamoxifen concurrently with adjuvant chemotherapy halves the benefit of the chemohormonal combination compared to giving tamoxifen after completion of chemotherapy in postmenopausal patients with node-positive, hormone-receptor positive breast cancer (ASCO abstract 143). These initial results from North American Breast Intergroup Trial 0100 (INT 0100, SWOG 8814), presented by Kathy S. Albain, MD, are expected to establish a new standard of care for treating breast cancer in postmenopausal women.
Based on a median follow-up of 8.5 years, the intergroup data showed that giving tamoxifen concurrently with chemotherapy reduces both disease-free survival and overall survival compared to giving chemotherapy followed by tamoxifen.
"Concurrent chemotamoxifen may result in suboptimal benefit from this or similar chemotherapy programs, potentially cutting efficacy by as much as 50%. The results of INT 0100 support a new practice standard of starting adjuvant tamoxifen after chemotherapy is completed," Dr. Albain said. She is the trial’s lead investigator as well as director of breast cancer research and co-director of the Breast Care Center at Loyola University’s Cardinal Bernardin Cancer Center in Chicago.
Researchers had suspected that tamoxifen might interfere with the effects of cytotoxic chemotherapy because many currently used antineoplastic agents are cell-cycle dependent. Tamoxifen freezes tumor cells in the mitotic pathway, making them less vulnerable to cytotoxic agents.
Objectives and Stratification
Dr. Albain said that the first objective of this trial was to determine whether adding tamoxifen to anthracycline-based adjuvant therapy with CAF (oral cyclophosphamide [Cytoxan, Neosar)/doxorubicin [Adriamycin]/fluorouracil) was superior to tamoxifen alone for treatment of breast cancer in postmenopausal women with node-positive and hormone-receptor positive disease. "Last year we reported a significant disease-free survival and overall survival advantage for the combination," she said.
The second objective, addressed in this year’s presentation, was to determine whether CAF followed by tamoxifen (CAF-T) was more effective than concurrent CAF-tamoxifen therapy (CAFT).
The study enrolled 1,477 postmenopausal women whose tumors were estrogen-receptor positive and/or progesterone-receptor positive and who had pathologic N1 or N2 lymph node status if the clinical stage was N0 or N1. "These patients had no distant metastases identifiable by enzymes, chest radiograph, or bone scans," Dr. Albain said. Thirty-two percent of patients were at least 65 years of age, and 13% were 70 or older. Forty-two percent of patients had 4 or more positive nodes.
Stratification was by nodes (1-3 vs 4 or more), progesterone receptor positive (PR+) vs progesterone receptor negative (PR-), and time from surgery to study registration of 6 weeks or less vs 6 to 12 weeks. The study was powered to detect a 33% improvement of sequential CAF-T over concurrent CAFT. One-sided testing was planned to determine statistical significance because the hypothesis was that sequential treatment was better.
The investigators randomized 361 women to 5 years of tamoxifen alone, 566 to sequential CAF-T, and 550 to concurrent CAFT. CAF was given every 4 weeks for 6 cycles.
Both Combinations Better
Both combination regimens were better then tamoxifen alone. Disease-free survival (DFS) at 8 years was 55% with tamoxifen alone but 62% with concurrent CAFT and 67% with CAF followed by tamoxifen (P = .004). The relative improvement in disease-free survival compared to tamoxifen alone was 44% for CAF followed by tamoxifen but dropped to 23% when the drugs were given together (see Figure 1).
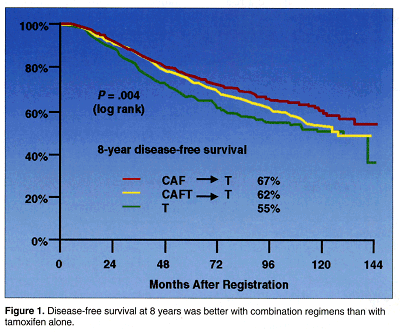
With regard to overall survival, 73% of patients treated with CAF-T were alive at 8 years, compared to 71% of patients treated with concurrent CAFT and 67% of patients treated with tamoxifen alone (P = .23) The relative improvement compared to tamoxifen alone was 25% with CAF followed by tamoxifen, vs 16% when the drugs were given together.
"There is now a 12% absolute improvement in DFS for the sequential arm compared to tamoxifen alone. The absolute difference is 6% for overall survival with sequential CAF-T compared to tamoxifen alone," Dr. Albain said. Concurrent CAFT produced a 7% improvement in DFS compared to tamoxifen alone.
Concurrent CAFT produced a 23% improvement in DFS and 16% improvement in overall survival compared to tamoxifen alone.
"For both disease-free and overall survival, concurrent tamoxifen reduced the effectiveness of chemotherapy by almost 50%," she concluded. In the Cox multivariate model, the confidence intervals did not include 1-0, which gives added robustness to the one-sided test, she noted.
Late-Appearing Events
"Even though this was a node-positive cohort, INT-0100 was marked by late-appearing events and benefits," Dr. Albain said. "The DFS advantage to combined treatment compared to tamoxifen alone appeared at approximately year 4. Three years later the survival benefit emerged. The DFS advantage for the sequential vs the concurrent arm appeared at year 8. It is possible that a significant advantage for survival will also emerge for the sequential arm if the same pattern occurs. Events occurred much later than predicted in this population, and there were more late events in the concurrent arm. Even more follow-up time is required to determine if these curves will continue to separate."
There were no differences in grade 2, 3, or 4 acute toxicities between the CAF-T and CAFT arms. Unlike previous reports, this study found no increased incidence of thromboembolic events associated with concurrent CAFT.
Dr. Albain concluded that after adjusting for other prognostic factors, delaying tamoxifen until the completion of CAF produces an 18% improvement in DFS compared to giving the drugs concurrently. "This results in a 12% absolute benefit over tamoxifen alone," she said.
"The results of INT-0100 support a new practice standard of starting adjuvant tamoxifen after chemotherapy is completed." She advised physicians who have patients presently on a concurrent chemotherapy/tamoxifen regimen to "stay the course" but pointed out that patients who opt to stop tamoxifen until they complete chemotherapy are likely running no major risk.
One-Sided Test Questioned
Discussant Melody A. Cobleigh, MD, of Rush Presbyterian St. Luke’s Medical Center in Chicago said, "These studies are so important in terms of standard of care that the ASCO health outcomes research committee might want to consider issuing a position paper."
Dr. Cobleigh raised questions about the use of the one-sided test of significance, which assumes that concurrent CAFT is the standard of care and that sequential CAF-T would be significant only if it were better. "A two-sided test would be more appropriate because there is no standard therapy and no way of knowing in advance which regimen would be better," she said. Reworking the data using a two-sided test would eliminate the statistical significance of the difference in DFS for sequential vs concurrent treatment (P = .06). Adjusting for contributing variables decreased the DFS difference even further, to P = .09 and the survival difference to P = .46.
Dr. Cobleigh said that remaining questions include the influence of HER2, estrogen receptor levels, and other factors on sequential vs concurrent therapy, whether the conclusions of this study with regard to sequential vs concurrent scheduling can be generalized to other regimens such as those including carboplatin (Paraplatin) or paclitaxel (Taxol), and whether similar effects will be seen with the aromatase inhibitors.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.