Single-Agent Sequential Rx May Be Reasonable for Metastatic Breast Cancer
ORLANDO-With an ever-expanding list of active agents for metastatic breast cancer and even more potential combinations, choosing the best therapy for each patient can be a challenge, according to Clifford Hudis, MD, chief of the
ORLANDOWith an ever-expanding list of active agents for metastatic breast cancer and even more potential combinations, choosing the best therapy for each patient can be a challenge, according to Clifford Hudis, MD, chief of the Breast Cancer Medicine Service at Memorial Sloan-Kettering Cancer Center. But Dr. Hudis makes a case for weighting the decision toward single-agent sequential therapy and for keeping quality of life at the forefront of the decision-making process.
Speaking at an industry-sponsored symposium held in conjunction with the 38th Annual Meeting of the American Society of Clinical Oncology, Dr. Hudis prefaced his discussion with a question often asked by patients: "If we can’t cure metastatic breast cancer, why do we treat it with chemotherapy, with all its side effects, both large and small?"
The answer, he said, is that "we know we can palliate breast cancer. In some cases, we can prolong survival, and that’s always remarkable when we see it. In many cases, we can show prolonged time to progression, which does, I believe, map to quality-of-life improvements. But quality of life really is the bottom line."
The approach to palliation of any metastatic cancer is twofold, he said. "You reduce tumor burden, but at the same time, you have to be very cognizant of any negative effect on quality of life that the treatment may cause."
With these assertions as a backdrop, Dr. Hudis reviewed the "largely overlapping first-line activity" of commonly used agents. He suggested that using response as the sole arbiter of benefit may not be the most optimal approach and that the order in which these agents are applied as first-, second-, or third-line therapy may not make a significant difference.
Moreover, he maintained that in many instances sequential therapy can be a better choice than combination therapy.
Dr. Hudis cited several studies of combination vs single-agent therapies in which the combinations were slightly more active in terms of response rate but showed no statistically significant effect on time to progression or overall survival. Quality of life, however, was better with single-agent regimens, he said.
"What this suggests," Dr. Hudis said, "is that at the end of the day when we look at quality of life, choosing agents that produce somewhat lower response rates may well be justified."
Capecitabine in Sequential Therapy
Capecitabine (Xeloda) satisfies the treatment goals for metastatic breast cancer, Dr. Hudis said, with its documented efficacy and little cost to quality of life. Moreover, he believes that capecitabine has potential as a single-agent therapy.
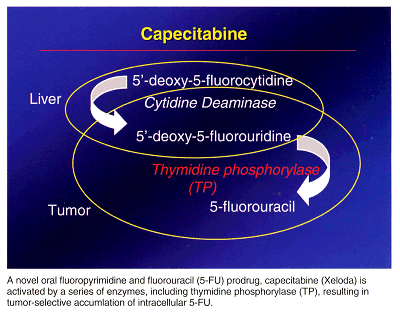
A novel oral fluoropyrimidine and fluorouracil (5-FU) prodrug, capecitabine is activated by a series of enzymes, including thymidine phosphorylase, resulting in tumor-selective accumulation of intracellular 5-FU (see figure below). The drug’s efficacy has been established in a variety of dos-ages and schedules, Dr. Hudis noted, including as a single-agent therapy in patients previously treated with anthracyclines and taxanes.
The drug’s dose-limiting toxicities are similar to those of infusional 5-FU, including hand-foot syndrome and diarrhea. Fatigue and cytopenia are a little more common with the oral drug, whereas stomatitis is more common with infusional 5-FU.
Dr. Hudis cautioned that capecitabine interacts with warfarin and that patients who are taking both drugs will have a prolonged prothrombin time, making frequent monitoring a must. This interaction is a particular concern for patients older than age 60, he added, and the problem is not related to the presence or absence of liver metastases.
In support of capecitabine’s potential as a single-agent therapy, Dr. Hudis cited a subset analysis of a phase III trial of docetaxel plus capecitabine vs docetaxel alone conducted by O’Shaughnessy et al, which was discussed at the symposium and subsequently published in the June 15, 2002, issue of the Journal of Clinical Oncology.
The analysis looked at 164 patients treated with single-agent docetaxel who went on to another single-agent therapy. Compared with patients given any agent but capecitabine, the 46 who received capecitabine had a hazard ratio for death of 0.5. The median survival of the capecitabine-treated patients was 21 months vs 12.3 months for those on any other drug.
Acknowledging that the subset analysis is "very exploratory and should be taken with a huge grain of salt," Dr. Hudis said, "this does suggest that maybe if you follow single-agent docetaxel with single-agent capecitabine, you get the same overall benefit as if you gave them together."
Another possibility under studyone that again appears to be supported by the O’Shaughnessy et al phase III trialis that the FDA-approved capecitabine dose, 1,250 mg/m² twice a day for 14 days, might be reduced without compromising efficacy.
Adjuvant Setting
Dr. Hudis reported that capecitabine is being moved into the adjuvant setting in a new Cancer and Leukemia Group B trial (CALGB 49907). The trial will evaluate single-agent capecitabine in a reduced dose (1,000 mg/m²twice a day for 14 days) vs either standard Adriamycin/cyclophosphamide (AC) or standard cyclophosphamide/methotrexate/5-FU (CMF) among women age 65 or older with high-risk breast cancer (either node negative and ER/PR negative or node positive).
In summary, Dr. Hudis said, capecitabine is clearly convenient, effective, and safe, and he expressed his "great faith in capecitabine’s ability to palliate stage IV disease at minimal cost to quality of life."