Diffuse Large B-Cell Non-Hodgkin Lymphoma in the Very Elderly: Challenges and Solutions
In this review, we critically analyze clinical trials that were specifically designed for the very elderly, and we discuss the challenges encountered by investigators who are conducting studies in this patient population. We conclude by proposing an algorithm to help clinicians determine the optimal therapeutic strategy for treatment of DLBCL in very elderly patients.
Diffuse large B-cell non-Hodgkin lymphoma (DLBCL) is a disease of the elderly, but our current guidelines and treatment paradigms for this disease are based on studies that have mainly enrolled younger patients. Because the number of people living beyond the age of 80 increased by more than 250% between 1960 and 2000, and since it is expected that the population over the age of 75 will triple by 2030, understanding how these elderly patients should be treated is paramount to improving outcomes for this potentially curable lymphoma. In this review, we outline the scope of the problem; we define “the elderly” and identify challenges in assessing this patient population. We also summarize pivotal studies that have been conducted in these elderly patients and suggest an algorithm to aid clinicians in making treatment decisions when faced with DLBCL patients older than 80.
Introduction
Life expectancy has dramatically increased over the past 4 decades, with the number of persons over the age of 80 years increasing by more than 250% between 1960 and 2000.[1] By 2015, this age group is expected to increase by another 50%.[2] By 2030, the number of people older than 75 years will have tripled, and the subgroup of persons 85 years of age or older will have doubled in that same period.[3] Diffuse large B-cell non-Hodgkin lymphoma (DLBCL) is the most commonly diagnosed non-Hodgkin lymphoma (NHL) in Western countries. Almost one-third of newly diagnosed patients are over the age of 75.[4] While DLBCL is a potentially curable malignancy, evidence suggests that elderly patients do worse than their younger counterparts.[5] These inferior outcomes may reflect undertreatment resulting from oncologists’ perception that elderly patients are unable to tolerate aggressive therapy. Also, comorbidities that are common in the elderly might preclude the use of curative regimens that predispose some patients to life-threatening complications. In addition, DLBCL in elderly patients may be biologically and molecularly different from DLBCL in younger patients.[6] Elderly patients are more frequently diagnosed with an immunoblastic variant of DLBCL than are younger patients; this variant, which is often a marker of the ABC genotype, is known to carry a poor prognosis.[7] Also, the activated B-cell–type DLBCL and the Epstein-Barr virus (EBV)-related lymphomas are more common in elderly patients; both histologies portend a worse outcome.[8-10]
These perceptions and observations have minimized the participation of elderly patients in the clinical trials that have shaped our current therapy of DLBCL, and have raised the question of how best to treat elderly patients with DLBCL.
In this review, we critically analyze clinical trials that were specifically designed for the very elderly, and we discuss the challenges encountered by investigators who are conducting studies in this patient population. We conclude by proposing an algorithm to help clinicians determine the optimal therapeutic strategy for treatment of DLBCL in very elderly patients, defined here as those over the age of 80 years.
What is elderly?
While advanced age is considered to be an adverse prognostic factor in patients with DLBCL, the definition of “elderly” has been arbitrarily arrived at. Since the median age of newly diagnosed DLBCL patients approaches 70 years,[4] defining “elderly” as “above 60 years of age” is inadequate. The International Prognostic Index (IPI) classifies patients over age 60 as elderly, but the IPI relied on clinical trials that included very few patients over the age of 80.[11] In addition, this classification was introduced before the era of chemoimmunotherapy and prior to the significant improvement in supportive care measures. Some have defined “elderly” as the age cutoff beyond which stem-cell transplantation is contraindicated-a suboptimal definition since patients over the age of 70 with good performance status may undergo transplantation in the current era.[12,13] Furthermore, several studies have challenged the definition of “elderly” as patients over the age of 60. Advani et al compared the performance of the IPI, the age-adjusted IPI, and the revised IPI (R-IPI) against the elderly IPI (E-IPI, which uses an age cutoff of 70 years) in 267 patients older than 60 years who were treated with rituximab (Rituxan) and CHOP (cyclophosphamide, vincristine, doxorubicin, and prednisone).[14] In this small dataset, the E-IPI was more predictive of outcomes. In a German study of elderly patients with DLBCL, the investigators suggested that 75 should be the age cutoff for “elderly,” as the outcome in those older than 75 was significantly inferior to that in patients younger than 75.[15] In the absence of large prospective comparative trials, the National Comprehensive Cancer Network (NCCN) classifies elderly patients into “young old” patients (aged between 65 and 75 years); “old” patients (aged 76 to 85 years), and “oldest old” patients (aged > 85 years).[16] We propose shifting away from a definition of “elderly” based on age alone in favor of a system that comprehensively assesses each patient individually for fitness for therapy.
Challenges in Assessing and Treating the Elderly
Performance status
The Karnofsky score and the Eastern Cooperative Oncology Group (ECOG) scales have routinely been used in clinical trials to assess performance status (PS) in individual patients. While most oncologists rely on one or the other of these scales in their decision making regarding the institution of aggressive cytotoxic therapies, neither scale accounts well for comorbidities nor do they comprehensively assess patients’ functional status. Despite its limitation, PS has been incorporated in risk assessment as part of the IPI and the age-adjusted IPI.[17] Retrospective analysis of a large data set of DLBCL patients over the age of 80 demonstrated that patients’ ability to perform their activities of daily living (ADLs), as opposed to their ECOG-PS, is more predictive of overall survival (OS) and progression-free survival (PFS).[18] ADLs are basic skills that patients need to maintain their independence at home, such as the ability to bathe, dress, feed themselves, maintain continence, and avoid falls.[19] Instrumental ADLs (IADLs) assess patients’ ability to do such things as use the telephone, use transportation, go shopping, and manage finances.[20] Many patients diagnosed with malignancies have difficulties with ADLs and IADLs despite an ECOG-PS of 0, 1, or 2; however, choices of therapies have often relied on the PS as opposed to comprehensive measures of functionality.[21,22] Maione et al showed that values of the baseline quality of life (P = .0003) and the IADL score (ie, the number of IADLs for which the patient was dependent on outside help; P = .04) were associated with significantly better prognosis, whereas the ADL score (P = .44) and the Charlson Comorbidity score (Table 1 (P = .66) had no prognostic value in over 500 elderly lung cancer patients. An ECOG-PS of 2 (P = .006) and a higher number of metastatic sites (P = .02) also predicted a shorter OS.[23]
TABLE 1
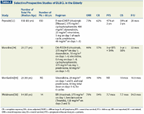
Charlson Weighted Comorbidity Index
a
In our opinion, deciding on a choice of therapy based on the PS without conducting a comprehensive geriatric assessment may underestimate elderly patients’ ability to tolerate treatment and might, in part, explain why they are offered less therapy. This, in turn, may deny them an opportunity for cure. The PS may complement a more comprehensive evaluation system.
Geriatric assessment
The comprehensive geriatric assessment (CGA) is a multidisciplinary evaluation of physical and psychological conditions that affect the choice of therapy for elderly patients with malignant disease. The CGA evaluates the nutritional status, cognitive skills, and comorbidities of elderly patients. Several groups have adapted CGA-based approaches in making therapeutic decisions. Examples of such decisions include omitting anthracyclines in patients with cardiac dysfunction, or omitting vincristine in those with neuropathy. Furthermore, using the CGA to help decide dose intensity and density of chemotherapy has resulted in excellent outcomes.[24] However, most studies that utilized the CGA included patients with a variety of cancers. Few trials were specifically designed for lymphoma patients. Nonetheless, these studies have shown that the domains evaluated in the CGA predict for morbidity and mortality in elderly cancer patients.[21] Incorporating the CGA into decision making would allow for the development of new endpoints in clinical trials that could specifically address quality of life and the impact of therapy on long-term physical and cognitive function. Rao et al demonstrated that elderly patients who were randomly assigned to inpatient geriatric assessment and management units experienced improved pain control and mental health scores.[25]
Tucci et al sought to determine whether the CGA could objectively identify elderly patients with DLBCL who could be effectively treated with anthracycline-based chemoimmunotherapy.[26] The CGA was performed in 84 consecutive DLBCL patients > 65 years of age who were treated with either curative or palliative intent according solely to clinical judgment. The outcomes of these patients were then analyzed according to both the treatment received and the results of the CGA. In total, 42 patients were classified as fit according to the CGA; these patients were younger (P < .0001) and had less frequent systemic symptoms (P = .03) compared with those classified as unfit by the CGA. The response rates (92.5% vs 48.8%; P < .0001) and median survival (not reached vs 8 months; P < .0001) were significantly better in the fit patients. Among the unfit patients, 20 had received curative therapy and 22 palliative therapy. The outcomes were similar irrespective of the type of treatment received (median survival, 8 vs 7 months; P < .05). Spina et al applied the CGA prospectively in a phase II study of 100 DLBCL patients over the age of 70 to assess the feasibility and efficacy of chemotherapy based on frailty.[27] Patients were divided into three groups (fit, unfit, and frail) using a modified CGA, and they then received rituximab and CHOP with dose adjustments based on comorbidities and the CGA.[27] The results support the use of the CGA as an efficient method of identifying elderly DLBCL patients who are fit enough to benefit from curative therapy and a way of sparing unfit patients unnecessary toxicities.
Because the CGA can be time-consuming, requiring up to 45 minutes to conduct for each patient,[28,29] less cumbersome assessment scales have been utilized.[24] Extermann et al assessed 24 readily available parameters in 518 patients starting chemotherapy for a variety of cancers.[30] The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score was constructed. Predictors of hematologic toxicity were the lymphocyte count, aspartate aminotransferase level, IADL score, lactate dehydrogenase (LDH) level, diastolic blood pressure, and the toxicity of the actual chemotherapy regimen. Predictors of nonhematologic toxicity were the hemoglobin level, creatinine clearance, albumin level, self-rated health, ECOG-PS score, Mini-Mental State Examination score, Mini-Nutritional Assessment score, and the toxicity of the actual chemotherapy used. The CRASH score distinguished several risk levels for severe side effects and was less time-consuming than a full CGA. However, the patients studied were heterogeneous and received various chemotherapy regimens, and few had lymphoid malignancies.[30] Hurria et al showed that incorporating the CGA, laboratory variables, tumor type, and patient and disease characteristics could predict chemotherapy toxicity in a variety of cancers for elderly patients. In this study, however, only 6% of the 500 studied patients had nonsolid tumors, and the mean age of treated patients was 73.[31] Predicting the toxicity of chemotherapy is critical in deciding which agents should be used and whether goals of therapy are safely achievable. Other scoring systems have been developed; one of the most commonly used is the Cumulative Illness Rating Scale for Geriatrics (CIRS-G).[32,33] While these scoring systems were not developed specifically for patients with lymphoma, their use in the general cancer population has provided evidence that they can affect outcomes.
In our opinion, decisions regarding treatment choice and goals in very elderly patients should rely more on these comprehensive predictive models than on the PS alone. However, prospective randomized studies in very elderly patients with DLBCL are needed to identify the most reliable system.
Comorbidities
Comorbidities are defined as chronic medical conditions that can affect a patient’s life span. Historically, the modified Charlson Comorbidity Index (see Table 1) has been used to estimate risk of mortality, based on pre-existing morbid conditions.[34] Janssen-Heijnen et al estimated that 80% of lymphoma patients older than 60 years have comorbid conditions compared with 48% of their younger counterparts.[35] The presence of comorbidities has predicted inferior OS in multivariate analyses. Several studies have demonstrated how comorbid conditions adversely affect outcomes in patients with prostate, breast, and colon cancers, and suggest that similar trends are likely to be seen in lymphoma patients.[36-38] It is also critical to evaluate the psychological state as an aspect of comorbidity, especially since older patients handle stressful illnesses poorly and their compliance is questionable.[39,40] Accordingly, routine assessment and comprehensive evaluation of comorbidity are needed prior to making a decision on the best therapeutic strategy in the very elderly.
Organ dysfunction
As patients age, organ dysfunction becomes more likely. Dysfunction may affect the kidneys, the liver, the heart, and bone marrow reserve.[41] Changes in organ function impact patients’ tolerance for therapy, pose significant challenges to the safe dosing of chemotherapeutics, and affect whether goals of care can be sustained.[42] Chemotherapy regimens that are designed to eradicate lymphomas and provide patients with an opportunity for cure might be prohibited in some patients with organ dysfunction. For example, platinum agents cannot safely be given in the presence of renal impairment. The critical administration of anthracyclines to patients with DLBCL cannot be attempted when cardiac dysfunction is present. Peripheral neuropathy could preclude administration of vincristine at full doses. Most elderly patients receive several nonchemotherapy medications to control their chronic systemic illnesses, and these may impact hepatic and renal functions.[43] Several studies have demonstrated continued decline in hematologic reserve as patients age. In fact, Marley et al showed that the decline in hematopoietic progenitor cell function may start at birth and continue throughout life.[44] If this is correct, one might expect more myelosuppression to occur in elderly patients than in younger patients in response to standard doses of chemotherapy. It is not surprising that studies have shown that advanced age is an independent risk factor for hospitalization and for the development of neutropenic fever.[45,46]
Retrospective Studies in Very Elderly DLBCL Patients
The difficulty of designing prospective studies in the population of very elderly patients with DLBCL has led researchers to analyze outcomes in the very elderly by conducting retrospective analyses. However, few of these reports included exclusively patients over the age of 80, since every analysis defined “elderly” differently. Hasselblom et al performed a retrospective study of 535 DLBCL patients (median age, 73 years) diagnosed in western Sweden between 1995 and 2000.[47] High IPI score and male sex had a negative impact on OS and PFS. Treatment with a curative intent was administered to only 376 patients (70%), without details as to why others did not receive curative therapy. However, this analysis was not restricted to patients > 80 years. These same investigators subsequently evaluated the outcome of a small cohort of patients > 80 who were treated in two sequential eras: before rituximab (1997 to 2000) and after rituximab (2006 to 2009).[48] Forty patients were identified with an improved 3-year PFS-and 30 patients with an improved OS-in the post-rituximab period compared to before (P = .01 for both). However, only 53% of patients received treatment with a curative intent. Thieblemont et al retrospectively studied 205 NHL patients over the age of 80 (median age, 83 years) to evaluate prognostic factors and outcomes.[49] Of these, 82 patients (40%) had DLBCL, 87% had at least one comorbidity, and 43% had a low Charlson Index score. Of the evaluable patients, 15% did not receive any treatment or received corticosteroids alone; 35% had surgery, radiotherapy, or mono-chemotherapy; 18% received poly-chemotherapy without anthracycline; and 32% received anthracycline-based therapy. Median OS was 1.3 years, and the main reason for death was disease progression (57%). Factors that were independently prognostic of survival were poor ECOG-PS (P < .0001) and high LDH level (P < .00001); comorbidities were not found to influence survival.
A larger multicenter retrospective analysis of 303 NHL patients ≥ 80 years treated between 1999 and 2009 was recently reported.[18] Of these patients, 127 (42%) had DLBCL. In this study, comorbidities were present in 80% of patients; 26% presented with at least one geriatric syndrome at initial diagnosis. Dementia was the most common geriatric syndrome, identified in 21 patients (26%), followed by osteoporosis in 18 (23%), depression in 16 (20%), falls in 15 (19%), and incontinence in 10 (12%). Additionally, 41 patients (14%) had a loss of at least one ADL; loss of ADLs was more common with aggressive compared with indolent NHLs (18% vs 6%, respectively; P = .002). At a 49-month median follow-up, 4-year PFS and OS for aggressive NHLs were 31% and 44%, respectively (stage I/II: PFS 53% and OS 66%; stage III/IV: PFS 20% and OS 32%; P < .0001 and P < .0002, respectively). Multivariate regression analysis identified two key factors that predicted inferior PFS and OS: lack of complete remission and loss of ADLs.[18] Exact details regarding the dose intensity and density used in treating these patients were not clearly identified.
Dose density and intensity proved important in providing patients with better outcomes. Of 109 patients with NHL > 80 years who were studied by Bairey et al, 66 (61%) had DLBCL; these patients were treated at three Israeli institutions between 1984 and 2004. While 18 patients out of the entire cohort of 109 patients (17%) received no therapy, 37 (34%) received radiation at some point (with or without chemotherapy) and 79 (72%) received chemotherapy. Only half of the chemotherapy-treated patients were able to complete the full prescribed program.[50] Complications were frequent, mostly hematologic toxicity, despite the administration of growth factors to one-third of patients. Patients who received more than 80% of the calculated combination chemotherapy dose (38% of patients) had a longer median survival than those who did not (72 vs 20 months). Patients who received any form of therapy had better OS than those who received no therapy (20 months vs 13 months), despite the fact that 26% of patients died from progressive disease.[50]
Van de Schans et al retrospectively investigated data on 419 patients with advanced-stage DLBCL, aged 75 years or older, who were diagnosed between 1997 and 2004 and were included in five regional population-based cancer registries in the Netherlands.[51] Only 46% of the patients received the standard therapy, and only 56% of these completed the planned full 6 cycles of treatment. Reasons for withholding chemotherapy were refusal by patient/family, poor PS, or estimated short life expectancy. Toxicities were observed in 67% of patients. Among patients older than 85 years, only 4% received standard optimal therapy. Among patients who received CHOP-like therapy, 73% of patients aged 80 to 84 years and 85% of patients aged > 85 years experienced hematologic toxicity. The 5-year survival rate for patients who received at least 6 cycles of CHOP-like therapy was 38%, compared with 22% in patients who received fewer than 6 cycles, and 4% in patients who received no chemotherapy.[51] While details on patients who were older than 80 years were not available (although they were available in those who were 75 to 80 years of age), these data suggest that, even in the elderly, full prescribed courses of therapy likely provide better outcomes than abbreviated courses when treating advanced disease.
Collectively, these studies demonstrate that very elderly patients are treated less frequently, experience more toxicity, and have less favorable outcomes, with worse OS and PFS, compared with younger patients. Importantly, progressive disease was a major cause of death in the very elderly. However, these studies have significant limitations. The definition of “elderly” was not uniform. Some studies reported on patients over age 65 while others suggested an age of 75 as a cutoff. Only a few studies included very elderly patients (> 80 years) exclusively. Furthermore, not all studies evaluated these very elderly patients using CGA and other geriatric scales, since the data needed to comprehensively conduct these evaluations were missing due to the retrospective nature of these analyses. Nonetheless, these trials paved the way for a few prospective phase II trials that have targeted this patient population.
Prospective Studies in Elderly Patients With DLBCL
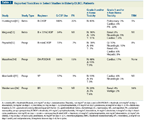
TABLE 2

Selective Prospective Studies of DLBCL in the Elderly
Since pivotal prospective DLBCL studies have generally defined “elderly” as 60 to 80 years, no uniform consensus exists as to how best to treat the very elderly (> 80 years). Some argue that replacing the R-CHOP (rituximab [Rituxan] plus CHOP) regimen with a less toxic non-anthracycline program is preferred, while others simply implement dose reductions of various R-CHOP components. In the absence of prospective randomized studies, it is unclear whether one strategy is superior to another. Importantly, few prospective studies (Table 2) have included exclusively patients older than 80 years, and all of these trials were small, phase II, single-institution studies.
In patients who had an ECOG-PS of 2 or less, Peyrade et al investigated the efficacy and safety of a decreased dose of CHOP (cyclophosphamide, 400 mg/m2; doxorubicin, 25 mg/m2; vincristine, 1 mg; and prednisone, 40 mg/m2) with a conventional dose of rituximab (375 mg/m2) in 150 DLBCL patients over the age of 80 (median age, 83 years).[52] Advanced-stage disease was present in 75% of treated patients. After a median follow-up of 20 months (range, 0 to 45 months), median OS was 29 months (95% confidence interval [CI], 21 to upper limit not reached); 2-year OS was 59% (95% CI, 49%–67%). In multivariate analyses, OS was only affected by a serum albumin concentration of 35 g/L or less (hazard ratio, 3.2; 95% CI, 1.4–7.1; P = .0053). Median PFS was 21 months (95% CI, 13 to upper limit not reached), with a 2-year PFS of 47% (95% CI, 38%–56%). There were 58 deaths reported, 33 of which were secondary to lymphoma progression. Twelve deaths (8%) were attributed to treatment toxicity. The most frequent side effect was hematologic toxicity (grade ≥ 3 neutropenia in 59 patients; febrile neutropenia in 11 patients). Others have also looked at reducing the intensity of CHOP. Meguro et al treated 61 DLBCL patients over the age of 70 (15 patients > 80 years) with 70%-dose CHOP plus full-dose rituximab for 6–8 cycles.[53] When these patients were compared to younger counterparts (< 70 years) who were treated with full-dose CHOP, 3-year OS and PFS were similar between both groups, suggesting that the use of CHOP in elderly patients might demonstrate similar benefits despite lower dose intensity. Importantly, despite the dose reduction, elderly patients still experienced more adverse events and toxicities, which were mainly hematologic.
An alternative anthracycline-based program was investigated by Musolino et al when 23 patients (10 of whom were older than 80 years) received dose-adjusted infusional cyclophosphamide, doxorubicin, vincristine, and prednisone with rituximab (DA-POCH-R).[54] Treated patients had poor prognosis, defined as having at least two adverse risk factors based on the IPI. The overall response rate (ORR) was 90% (57% achieved a complete response[CR]), and the 3-year OS was 56%. Most adverse events were hematologic, specifically neutropenia in 48% of the patients. The number of patients over the age of 80 in this cohort is too small to specifically form a conclusion regarding their outcomes, however.
Non-anthracycline–based regimens were also studied. In the pre-rituximab era, Monfardini et al treated 30 frail patients with the combination of vinorelbine (25 mg/m2 on days 1 and 8) and prednisone (30 mg daily on days 1 through 8) every 3 weeks for 6 cycles.[55] The criteria for frailty were age older than 80 years, age older than 70 with three or more comorbidities of grade 3, or at least one comorbidity of grade 4 using the CIRS. The ORR was 40% (10% achieved a CR and 30% had a partial response [PR]), with 10 additional patients demonstrating stable disease. Three patients died of heart failure within 28 days of therapy. Median OS was 10 months. However, some patients in this study (24%) had different histologies, including mantle cell, peripheral T-cell, and follicular NHL. Weidmann et al studied the activity of bendamustine (Treanda) plus rituximab in 14 patients > 80 years of age (median age, 84 years) who were considered ineligible for R-CHOP or who refused aggressive treatment.[56] Ineligibility for R-CHOP was left to the investigator’s discretion without uniform criteria. Of 13 evaluable patients, 7 (54%) achieved CR, and 2 (15%) had a PR, with a median OS of 7.7 months. The major toxicity was also hematologic: 17% of the patients developed grade 3 neutropenia and 6% had grade 4.
Collectively, these studies demonstrate that anthracyclines can still be used in some patients and that other non-anthracycline–based regimens also have activity. Whether omitting anthracyclines in the very elderly has an adverse impact on their outcomes is yet to be seen and will depend on the results of future large prospective studies. Also, whether patients older than 80 years can be treated in a fashion similar to those between the ages of 70 and 80 remains unclear.
Practical Questions in the Very Elderly
What are the goals of therapy?
The first clinical decision facing physicians who treat very elderly patients with DLBCL is whether the goal of therapy should be palliative or curative. Anthracycline-based chemo-immunotherapy programs have provided DLBCL patients with cure rates exceeding 50% depending on their pre-defined risk-stratification profile.[4] In younger patients, regimens that omitted anthracyclines compromised cure,[4] and full-dose intensity is considered to optimize chances for cure.
Deciding on the goals of therapy requires a full assessment of the patient’s physical condition and an accurate estimate of the life expectancy, as recent analysis has suggested that the overall expectation of life at birth is 77.7 years, with some variability between races and sexes.[57,58] If comorbidities and geriatric assessment suggest that patients cannot tolerate curative regimens, reduced-dose standard chemotherapy or non-anthracycline regimens may still be appropriate with a palliative intent. Some elderly patients present with a decline in their PS that is solely due to their lymphoma. In such instances, starting with a pre-phase treatment might be appropriate to improve patients’ functional status. The German Lymphoma Study Group suggested treating patients who present with ECOG PS of 2 or higher with 7 days of prednisone and 1 mg of vincristine before the first chemotherapy cycle.[59] This might improve the patients’ PS and allow for safer chemotherapy administration. If a patient’s status improves, goals of therapy can then be modified.
After assessing organ function (specifically cardiac ejection fraction), comorbidities, PS, and life expectancy, the physician should formulate an opinion as to whether an individual patient can tolerate full-intensity therapy. If full-dose intensity and density are utilized, careful attention to supportive care measures is critical. It is our opinion that these patients should be seen frequently and be carefully monitored for side effects and adverse events. Frequent monitoring of cardiac function in those who receive anthracyclines is likely to minimize the chances of cardiac toxicity. We routinely reassess ejection fraction by performing an echocardiogram or a multigated acquisition (MUGA) scan after the 4th cycle of anthracycline, although some have suggested more frequent monitoring.[60,61]
What is the best treatment for very elderly patients with DLBCL?
Generally, managing patients with DLBCL depends on the disease stage. In the very elderly, the optimal approach might be limited by the factors that have been discussed above. Historically, DLBCL patients with stage I or II disease have been managed with an abbreviated course of chemoimmunotherapy using R-CHOP, followed by involved-field radiation.[62] The debate over whether radiotherapy remains an essential part of treatment was ignited by long-term follow-up data from a randomized study showing late relapses in those who received radiation.[63,64] This debate was rekindled when Bonnet et al demonstrated that adding radiation to CHOP chemotherapy provided no survival benefit in DLBCL patients with good-risk limited-stage disease.[65] However, very elderly patients might have a limited life expectancy, in which case less cytotoxic therapy would be advisable to minimize the toxicity and adverse events associated with more chemotherapy cycles. If R-CHOP is judged to be overly toxic, alternative chemotherapy programs, as discussed previously, or alternative dosing schedules of R-CHOP can be implemented. In the absence of clear guidelines, the decision of how to proceed is usually left to the physician’s discretion. We favor using reduced doses of anthracyclines, as opposed to non-anthracycline–based programs-but always favor enrolling patients into prospective clinical trials when possible. Older studies that used radiation alone in DLBCL patients provided elderly patients with a cause-specific survival of 31% to 57% at 10 years.[66] When radiotherapy is used, doses of 30 Gy are usually utilized.[67]
Patients with advanced disease represent a true management challenge, since they require prolonged courses of systemic therapy. R-CHOP has clearly demonstrated superiority to standard CHOP in all patient categories, but trials that looked at the very elderly have been limited, as discussed above.[15,68-70] Prior to the widespread use of rituximab, studies that looked at the effect of adding etoposide to standard CHOP showed improved outcomes in elderly patients aged 60 to 80 years.[59] While the German Lymphoma Study Group advocates R-CHOP given in a dose-dense fashion,[40] studies that compared dose-dense R-CHOP to standard R-CHOP failed to show improvement in outcomes with the dose-dense regimen.[71,72] We encourage the very elderly to be enrolled in prospective clinical trials specifically designed for this patient population. In the absence of clinical studies, we recommend a dose-reduced R-CHOP or a non-anthracycline–based program in those with compromised cardiac function or other comorbidity. In fit patients with adequate cardiac reserve, full-dose R-CHOP is recommended with frequent response assessment so that unnecessary additional chemotherapy cycles can be withheld.
What about supportive care and CNS prophylaxis?
Current guidelines recommend primary prophylaxis with filgrastim (granulocyte colony-stimulating factor [G-CSF]) or pegfilgrastim (Neulasta) when the incidence of neutropenic fever after cytotoxic therapy is estimated at 20%.[73,74] Several studies have failed to demonstrate a survival benefit when growth factors are used in lymphoma patients.[75-77] Nonetheless, their use is associated with less hospitalization, shorter duration of neutropenia, fewer infectious complications, and possible healthcare cost savings.[46] Because bone marrow reserve decreases with advanced age, elderly patients are expected to be at increased risk for neutropenic fever, even if they are considered functionally fit.[78] Since the risk of infectious complications is greatest after the first cycle of therapy,[46] we recommend growth factors prophylactically from the onset of treatment. Furthermore, we recommend implementing prophylactic fluoroquinolones whenever the absolute neutrophil count is less than 500/μL, in a manner similar to that suggested by the German Lymphoma Study Group.[40] While no prospective studies have demonstrated the benefit of using trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis, we routinely advise elderly patients to take TMP-SMX prophylactically in order to minimize the risk of Pneumocystis jiroveci infection, especially when prolonged courses of corticosteroids are anticipated. Monitoring for other toxicities that can be encountered in elderly patients (Table 3) is critical in order to optimize outcome. For some elderly patients who receive 5 days of prednisone as part of the CHOP regimen, stopping corticosteroids abruptly might cause significant fatigue and tiredness. For these patients, we recommend a slow taper of the cortocosteroids to avoid withdrawal toxicity.
TABLE 3
Reported Toxicities in Select Studies in Elderly DLBCL Patients
Unfortunately, approximately 5% of patients with advanced-stage DLBCL develop a secondary CNS recurrence followed invariably by rapid death.[79] Traditionally, patients with testicular involvement and those who have an elevated LDH level and involvement of one extra-nodal site have an increased risk of CNS disease and are usually offered prophylaxis.[80] Efforts to reduce the incidence of CNS relapse have yielded conflicting results. Adding to the controversy is the fact that over half of patients who present with CNS relapse also have systemic disease.[22] A population-based study by Villa et al suggested reduction in CNS relapse after the introduction of rituximab into DLBCL treatment programs.[81] While Abramson et al showed that intrathecal methotrexate can be administered safely with systemic R-CHOP,[82] and also showed that intrathecal methotrexate decreased CNS relapse, others were unable to duplicate these results.[83] Also, Bernstein et al reported on 20-year follow-up data in 899 patients with advanced-stage DLBCL, showing a cumulative incidence of CNS relapse of 2.8%.[84] CNS relapse occurred early, with the majority of relapsing patients manifesting disease during chemotherapy or within 6 months of completion. The number of extra-nodal sites and the IPI were predictive of CNS relapse. Importantly, there was no significant benefit of CNS prophylaxis in patients with bone marrow involvement at diagnosis. These controversial findings, coupled with the potential toxicity of intrathecal therapy, lead us to suggest against routine use of prophylaxis. In high-risk patients, systemic administration of intermediate-dose methotrexate seems to be as effective and less toxic and can be considered.[40]
FIGURE
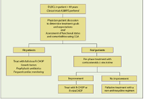
A Suggested Approach to Treating DLBCL Patients Over the Age of 80
Concluding Remarks
Elderly patients with DLBCL are a rapidly growing population that represents a therapeutic challenge. Guidelines on specifically how these patients should be treated are lacking. We recommend implementing the CGA as a measure to decide the best treatment strategy. The CGA should be followed by clear assessment of treatment goals, which requires open discussion with the elderly patient and his or her family. Regardless of treatment goals, we suggest preferential enrollment of elderly patients into clinical trials when available. When studies are not available and when a curative approach is deemed appropriate, we recommend R-CHOP at the full dose, with primary prophylaxis using G-CSF, antibiotics, and aggressive supportive measures in those who are found fit by the CGA (Figure). We suggest that patients who are deemed unfit or frail based on the CGA receive a pre-phase treatment with corticosteroids and vincristine. If PS and functionality improve, then either R-CHOP or R-miniCHOP (in which lower doses of the CHOP components of the regimen are used) is recommended. Those who do not improve despite pre-phase treatment are usually offered non-anthracycline–based therapy with palliative intent. Prospective clinical trials that are designed specifically for this patient population are in progress, and the results are eagerly awaited.
Financial Disclosure:Dr. Nabhan has received research grant support and honoraria from Genentech. The other authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol Biol. 2009;471:3-29.
2. Mora O, Zucca E. Management of elderly patients with hematological neoplasms. Ann Oncol. 2007;18(Suppl 1):i49-53.
3. Thieblemont C, Coiffier B. Lymphoma in older patients. J Clin Oncol. 2007;25:1916-23.
4. Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110:29-36.
5. Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22: 941-52,ix.
6. Thunberg U, Enblad G, Berglund M. Classification of diffuse large B-cell lymphoma by immunohistochemistry demonstrates that elderly patients are more common in the non-GC subgroup and younger patients in the GC subgroup. Haematologica. 2012;97:e3; author reply e4.
7. Ott G, Ziepert M, Klapper W, et al. Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood. 2010;116:4916-25.
8. Oschlies I, Klapper W, Zimmermann M, et al. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) Multicenter Trial. Blood. 2006;107:4047-52.
9. Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124-32.
10. Park S, Lee J, Ko YH, et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood. 2007;110:972-8.
11. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987-94.
12. Jantunen E, Canals C, Rambaldi A, et al. Autologous stem cell transplantation in elderly patients (> or =60 years) with diffuse large B-cell lymphoma: an analysis based on data in the European Blood and Marrow Transplantation registry. Haematologica. 2008;93:1837-42.
13. Bitran JD, Klein L, Link D, et al. High-dose myeloablative therapy and autologous peripheral blood progenitor cell transplantation for elderly patients (greater than 65 years of age) with relapsed large cell lymphoma. Biol Blood Marrow Transplant. 2003;9:383-8.
14. Advani RH, Chen H, Habermann TM, et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793): consideration of age greater than 70 years in an elderly prognostic index (E-IPI). Br J Haematol. 2010;151:143-51.
15. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105-16.
16. Balducci L. Management of cancer in the elderly. Oncology (Williston Park). 2006;20:135-43; discussion 44, 46, 51-2.
17. Ziepert M, Hasenclever D, Kuhnt E, et al. Standard international prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:2373-80.
18. Nabhan C, Smith SM, Helenowski I, et al. Analysis of very elderly (>/=80 years) non-Hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2012;156:196-204.
19. Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-9.
20. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-86.
21. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824-31.
22. Fields PA, Linch DC. Treatment of the elderly patient with diffuse large B-cell lymphoma. Br J Haematol. 2012;157:159-70.
23. Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865-72.
24. Merli F, Luminari S, Rossi G, et al. Cyclo-phosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly “fit” patients with diffuse large B-cell lymphoma: results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma. 2012;53:581-8.
25. Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci. 2005;60:798-803.
26. Tucci A, Ferrari S, Bottelli C, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115:4547-53.
27. Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist. 2012;17:838-46.
28. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998-2005.
29. Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104: 1133-63.
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377-86.
31. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457-65.
32. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130-7.
33. Waldman E, Potter JF. A prospective evaluation of the cumulative illness rating scale. Aging (Milano). 1992;4:171-8.
34. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-83.
35. Janssen-Heijnen ML, van Spronsen DJ, Lemmens VE, et al. A population-based study of severity of comorbidity among patients with non-Hodgkin’s lymphoma: prognostic impact independent of International Prognostic Index. Br J Haematol. 2005;129:597-606.
36. Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42-51.
37. Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41:2887-95.
38. Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433-40.
39. Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556-62.
40. Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103-10.
41. Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60: 120-32.
42. Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086-93.
43. Wynne HA, Cope LH, Mutch E, et al. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297-301.
44. Marley SB, Lewis JL, Davidson RJ, et al. Evidence for a continuous decline in haemopoietic cell function from birth: application to evaluating bone marrow failure in children. Br J Haematol. 1999;106:162-6.
45. Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003;98:2402-9.
46. Lyman GH, Morrison VA, Dale DC, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44:2069-76.
47. Hasselblom S, Ridell B, Nilsson-Ehle H, Andersson PO. The impact of gender, age and patient selection on prognosis and outcome in diffuse large B-cell lymphoma-a population-based study. Leuk Lymphoma. 2007;48:736-45.
48. Hasselblom S, Stenson M, Werlenius O, et al. Improved outcome for very elderly patients with diffuse large B-cell lymphoma in the immunochemotherapy era. Leuk Lymphoma. 2012;53:394-9.
49. Thieblemont C, Grossoeuvre A, Houot R, et al. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19:774-9.
50. Bairey O, Benjamini O, Blickstein D, et al. Non-Hodgkin’s lymphoma in patients 80 years of age or older. Ann Oncol. 2006;17:928-34.
51. van de Schans SA, Wymenga AN, van Spronsen DJ, et al. Two sides of the medallion: poor treatment tolerance but better survival by standard chemotherapy in elderly patients with advanced-stage diffuse large B-cell lymphoma. Ann Oncol. 2012;23:1280-6.
52. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460-8.
53. Meguro A, Ozaki K, Sato K, et al. Rituximab plus 70% cyclophosphamide, doxorubicin, vincristine and prednisone for Japanese patients with diffuse large B-cell lymphoma aged 70 years and older. Leuk Lymphoma. 2012;53:43-9.
54. Musolino A, Boggiani D, Panebianco M, et al. Activity and safety of dose-adjusted infusional cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy with rituximab in very elderly patients with poor-prognostic untreated diffuse large B-cell non-Hodgkin lymphoma. Cancer. 2011;117:964-73.
55. Monfardini S, Aversa SM, Zoli V, et al. Vinorelbine and prednisone in frail elderly patients with intermediate-high grade non-Hodgkin’s lymphomas. Ann Oncol. 2005;16:1352-8.
56. Weidmann E, Neumann A, Fauth F, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011;22:1839-44.
57. Arias E. United States life tables by Hispanic origin. Vital Health Stat 2. 2010;1-33.
58. Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1-40.
59. Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634-41.
60. Aapro M. SIOG (International Society of Geriatric Oncology) recommendations for anthracycline use in the elderly. Hematol Rep. 2011;3:e6.
61. Aapro M, Bernard-Marty C, Brain EG, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol. 2011;22:257-67.
62. Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin’s lymphomas. J Natl Compr Canc Netw. 2011;9:484-560.
63. Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21-6.
64. Miller T, LeBlanc M, Chase E, Fisher RI. Chemotherapy alone compared with chemotherapy plus radiotherapy for early stage aggressive non-Hodgkin lymphoma: update of the Southwest Oncology Group radomized trials. Ann Hematol. 2001;80:B154.
65. Bonnet C, Fillet G, Mounier N, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25:787-92.
66. Vaughan Hudson B, Vaughan Hudson G, MacLennan KA, et al. Clinical stage 1 non-Hodgkin’s lymphoma: long-term follow-up of patients treated by the British National Lymphoma Investigation with radiotherapy alone as initial therapy. Br J Cancer. 1994;69:1088-93.
67. Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100:86-92.
68. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040-5.
69. Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121-7.
70. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379-91.
71. Cunningham D, Smith P, Mouncey P, et al. R-CHOP14 versus R-CHOP21: Result of a randomized phase III trial for the treatment of patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma. J Clin Oncol 2011;29:Abstr 8000.
72. Delarue R, Tilly H, Salles G, et al. R-CHOP14 Compared to R-CHOP21 in elderly patients with diffuse large B-cell lymphoma: results of the interim analysis of the LNH03-6B GELA study. Blood. 2009;114:Abstr 416.
73. Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8-32.
74. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187-205.
75. Burton C, Linch D, Hoskin P, et al. A phase III trial comparing CHOP to PMitCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin’s lymphoma. Br J Cancer. 2006;94:806-13.
76. Doorduijn JK, van der Holt B, van Imhoff GW, et al. CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21:3041-50.
77. Osby E, Hagberg H, Kvaloy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood. 2003;101:3840-8.
78. Ziepert M, Schmits R, Trumper L, et al. Prognostic factors for hematotoxicity of chemotherapy in aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2008;19:752-62.
79. Ferreri AJ, Assanelli A, Crocchiolo R Ciceri F. Central nervous system dissemination in immunocompetent patients with aggressive lymphomas: incidence, risk factors and therapeutic options. Hematol Oncol. 2009;27:61-70.
80. van Besien K, Gisselbrecht C, Pfreundschuh M, Zucca E. Secondary lymphomas of the central nervous system: risk, prophylaxis and treatment. Leuk Lymphoma. 2008;49 (Suppl 1):52-8.
81. Villa D, Connors JM, Shenkier TN, et al. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21:1046-52.
82. Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116:4283-90.
83. Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113:3896-902.
84. Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516-the Southwest Oncology Group. J Clin Oncol. 2009;27:114-9.
85. Huntington SF, Talbott MS, Greer JP, et al. Toxicities and outcomes among septuagenarians and octogenarians with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone therapy. Leuk Lymphoma. 2012;53:1461-8.