Disseminated Intravascular Coagulation in Patients With Solid Tumors
This review summarizes the published data regarding the association of disseminated intravascular coagulation (DIC) with solid tumors, the laboratory diagnosis of DIC, the clinical presentation and types of tumors typically seen with DIC, and the pathophysiologic mechanisms involved; it also offers suggestions for management of DIC in patients with solid tumors.
Figure: Proposed Algorithm for Management of Patients With a DIC Score ≥ 5 and With Known or Suspected Solid Tumors
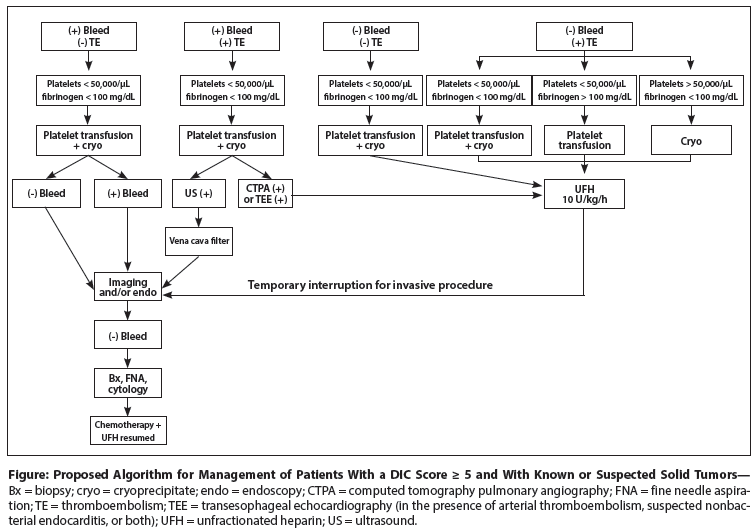
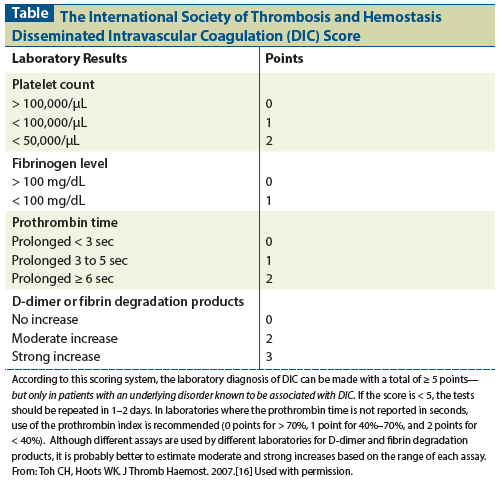
Table: The International Society of Thrombosis and Hemostasis Disseminated Intravascular Coagulation (DIC) Score
Disseminated intravascular coagulation (DIC) is an occasional complication of solid tumors, usually identified at the time of presentation because of excessive bleeding, thromboembolic complications, or abnormal laboratory test results. The latter include an unexplained low platelet count, a low fibrinogen level, an elevated D-dimer level, and a prolonged prothrombin time. Prompt diagnosis and treatment of the underlying malignancy can result in resolution of the DIC. Further, if the tumor is responsive to chemotherapy, a reasonable median survival can also result. Excessive bleeding at presentation can be managed with platelet transfusions, cryoprecipitate, and fresh frozen plasma. Thromboembolic complications can be managed with continuous intravenous heparin and supportive platelet transfusions; cryoprecipitate can be used whenever necessary to support platelet and fibrinogen levels. On occasion, when excessive bleeding and venous thromboembolism occur together, placement of a vena cava filter is required, along with the administration of platelets and cryoprecipitate.
Introduction
Disseminated intravascular coagulation (DIC) is a dynamic pathologic process triggered by activation of the blood coagulation pathways; it results in generation of excess thrombin within the intravascular space, sometimes extending to the extravascular space. Shortened survival (consumption) of several hemostatic factors, deposition of fibrin in the microcirculation, and activation of the fibrinolytic system follow. Lowered levels of hemostatic factors (platelets, factors V and VIII, and fibrinogen) and secondary fibrinolysis may result in excessive bleeding when the vasculature is damaged by vascular punctures, trauma, tumors, or surgery. In contrast, fibrin deposition may cause micro- or macrothrombosis, organ dysfunction, and sometimes a fragmentation type of hemolytic anemia (microangiopathic hemolytic anemia).
The causes of DIC can be classified into several groups of disorders: severe infections of any type, obstetric complications such as abruptio placenta, massive tissue necrosis such as in heat stroke, severe prolonged shock of any type, snake bite, large vascular disorders such as aortic dissection and large-vessel hemangioma (Kasabach-Merritt), major hemolytic transfusion reactions, and acute leukemias (particularly acute promyelocytic leukemia [APL]; DIC also not infrequently complicates acute lymphoblastic leukemia, and occasionally acute myeloblastic leukemia).[1,2] In addition to its almost universal occurrence in APL, DIC has also been occasionally reported in patients with solid tumors.[3] Several recent reviews[4-9] provide excellent summaries of the diagnosis and management of DIC in APL. This review will attempt to summarize the published data regarding the association of DIC with solid tumors, the laboratory diagnosis of DIC, the clinical presentation and types of tumors typically seen with DIC, and the pathophysiologic mechanisms involved; it will also offer suggestions for management of DIC in patients with solid tumors.
Background
The association between solid tumors and a thrombohemorrhagic disorder was first described by Trousseau in 1865.[10] This association was greatly expanded over the years, culminating in a 1977 review and summary by Sack et al of the thrombotic and excessive bleeding complications associated with neoplastic disease.[11] They found that the most common thrombohemorrhagic clinical manifestations associated with solid tumors were isolated venous thrombosis in 62%, “migrating” venous thrombosis in 53%, and excessive bleeding in 41%. In addition, some patients had nonbacterial endocarditis with or without arterial embolization, and a few had evidence of DIC. Thus, there was clearly a great deal of evidence that many malignancies were associated with an increased incidence of thromboembolic complications, excessive bleeding, and, in a small fraction, evidence of DIC.
The largest prospective study of DIC in patients with solid tumors was carried out by Sallah et al.[12] To be eligible for this study, patients had to be symptomatic, with excessive bleeding and evidence of superficial or deep vein thrombosis or pulmonary embolism. In addition, they had to have laboratory evidence of DIC, including at least three of the following hemostatic abnormalities: platelet count < 150,000/µL, fibrinogen level < 200 mg/dL, elevated D-dimer level, elevated fibrin degradation products, fragmented red cells on the peripheral blood smear, prolonged prothrombin time, or prolonged partial thromboplastin time. These are relatively broad criteria compared with the guidelines established by the International Society on Thrombosis and Haemostasis (ISTH) and other groups.[13-20] Using the ISTH criteria, Sallah et al made a diagnosis of DIC in 76 of 1,117 patients (6.8%). Further, of the patients with DIC, 76% had stage III/IV cancer; 59% presented with excessive bleeding, 34% with thrombosis, and 7% with both thrombosis and bleeding. The most common associated tumors originated from the lung, breast, prostate, colon, and rectum; this finding was not significantly different from the types of tumors in the 1,041 patients who did not have clinical or laboratory evidence of DIC. The only difference between the two cohorts of patients was older age (68 vs 60 years) and more frequent presence of liver metastasis (46% vs 24%) in the DIC group. In the multivariate analysis of the entire 1,117 patients, the only significant independent factors associated with DIC were older age, male gender, higher-stage cancer, breast cancer, and presence of necrosis in the tumor specimen. Management was variable. Some patients received anticoagulation with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH), and a few received antithrombin III concentrates. Patients with excessive bleeding were given various amounts of platelet transfusions, cryoprecipitate, and fresh frozen plasma. Treatment of the underlying malignancy was based on the patient’s performance status and prior therapy. Patients with advanced disease and DIC had a median survival of 9 months, compared with 14 months in those without DIC (P = .005). Interestingly, patients with stage I and II disease with DIC had a median survival of 16 months, compared with 44 months in those without DIC. Thus, the presence of DIC shortened survival significantly in both early- and late-stage disease. From this study, one can conclude that, although the patients with DIC had shortened survival, many did survive for a significant period of time from the onset of DIC; further, if the underlying tumor was treatable and the patient had a reasonable performance status, chemotherapy was probably helpful.
Sakuragawa et al also reported a large study of DIC in patients with solid tumors.[21] This was a multi-institutional study of the treatment of DIC of all causes (acute leukemia, solid tumors, sepsis, obstetric complications, etc), comparing LMWH (dalteparin) with UFH. Of the 126 patients with different underlying disorders enrolled, 47 had solid tumors. This was a double-blind study comparing a fixed dose of dalteparin (75 IU/kg) given by continuous intravenous infusion over 24 hours (ie, 3.1 IU/kg/h) with UFH 240 U/kg over 24 hours (ie, 10 U/kg/h). The anticoagulants were given for the first 5 days after admission. On occasion, the infusion was discontinued before the end of the 5 days. The “stopping criteria” were not defined. Diagnostic criteria for DIC and for entry into the study were those established by the Japanese Research Committee on DIC in the Ministry of Health and Welfare.[22] They included an underlying disease known to be associated with DIC, excessive bleeding, organ dysfunction, fibrin degradation product levels, platelet count, and prothrombin time. In order to make a diagnosis of DIC in the nonleukemia cohort, a score of ≥ 7 out of 13 was necessary. In addition, tests involving other, more sensitive indicators of DIC-including the presence of soluble fibrin monomer complexes, increased levels of thrombin–antithrombin III complexes, low levels of antithrombin III, and increased levels of plasmin–alpha 2–plasmin inhibitor complexes-were performed. Several hemostatic studies were done during the 5-day period when the patients were receiving anticoagulant therapy. There were 61 patients in the LMWH group and 63 in the UFH group. Of these 124 patients, 47 had solid tumors and 49 had acute leukemia, which was APL in 21. The remainder of the patients had other disorders associated with DIC. Not all of the data were analyzed according to the underlying diseases. However, DIC scores progressively improved over the 5 days of treatment with both LMWH and UFH. Bleeding symptoms were improved in 44 patients, unchanged in 42 patients, and worse in 13 patients. In the patients with solid tumors, overall status was judged to be improved in 25, unchanged in 14, worse in 6, and difficult to judge in 2 patients. Interestingly, in the patients with APL, overall improvement occurred in 17; clinical status was unchanged in 1 patient and worsened in only 3 patients. In contrast, in the other acute leukemias, the clinical status was improved in 20, unchanged in 2, worse in 5, and unable to be judged in 6 patients. Thus, it was clear that, in patients with acute leukemia (who have the most severe bleeding problems associated with DIC), low-dose UFH and LMWH had very few adverse effects compared with those in patients with solid tumors. Interestingly, LMWH performed somewhat better than UFH, but the difference between the two cohorts was not significant.
A multi-institutional retrospective study of 68 patients with metastatic gastric adenocarcinoma (80% signet ring cell type) associated with DIC compared chemotherapy with best supportive care.[23] In the 19 patients who received chemotherapy (28%), the median overall survival was 61 days, whereas in the 49 patients who received best supportive care (72%), the median survival time was only 9 days (P < .001). Although no details were given regarding the specific management of the DIC, “DIC-related symptoms” improved in 8 of 19 patients (42%). This study and a few other smaller studies in patients with DIC associated with gastric or colorectal adenocarcinoma suggest that chemotherapy may be beneficial in some of these patients.[24-31]
Guidelines for Making the Diagnosis of Disseminated Intravascular Coagulation
Although it has been stated that no one laboratory test can rule in or rule out the diagnosis of DIC, a negative D-dimer test result has a very strong negative predictive value. In contrast, there is no single laboratory test or combination of tests that can substantiate an unequivocal diagnosis of DIC. Thus, several scoring systems have been proposed by national societies and by the Scientific and Standardization Committee on DIC of the ISTH.[13-20] These scoring systems have been compared with one another and with the one established by the ISTH, and there are only minor differences.[20] The ISTH guidelines, which are based on a combination of simple, rapid tests readily available in all clinical laboratories, clearly provide substantial evidence for DIC in the context of an appropriate clinical setting (ie, in the presence of an underlying disorder associated with DIC).[14-16,32] This well-established scoring system and set of guidelines are shown in the Table.
According to the ISTH guidelines, a laboratory diagnosis of DIC can be made when a patient has a total score of ≥ 5, but only in patients with an underlying disorder known to be associated with DIC. If the score is < 5, then the tests should be repeated in 1 to 2 days. For laboratories that do not report the prothrombin time in seconds, use of the prothrombin index is recommended, with 0 points for values > 70%, 1 point for 40% to 70%, and 2 points for < 40%. Since different assays are used by different laboratories to measure D-dimer and fibrin degradation products, it is probably better to estimate moderate and strong increases for each based on the range of the assay used.
Other society guidelines have been published that differ from the ISTH guidelines in minor ways.[17-19] Although the ISTH DIC score was shown in one study to have a sensitivity of 93% and a specificity of 98%, the majority of patients in that study had some form of sepsis, major trauma, or major surgery. None were characterized as having malignant disease.[14] The ISTH DIC score has never been studied in a large cohort of patients with solid tumors.
None of the published guidelines give points for a prolonged partial thromboplastin time or for evidence of fragmentation hemolytic anemia. Moreover, the ISTH guidelines and most of the other guidelines do not take into account defects in protein synthesis (liver dysfunction, vitamin K deficiency, etc) or other causes of thrombocytopenia (marrow replacement by tumor or effect of chemotherapy). In addition, if liver function is relatively normal, the rate of fibrinogen synthesis (as an acute-phase reactant) may be increased enough to equal or exceed its catabolic rate, so that fibrinogen levels are within normal limits or even occasionally increased. Because DIC is usually a chronic, relatively slow process in patients with solid tumors, the fibrinogen level may be normal or even occasionally elevated. Thus, in patients with solid tumors in whom bone marrow and liver function are usually normal, the platelet count may be low-normal or only slightly decreased and the fibrinogen level may be normal or even increased. The latter finding, along with an elevated D-dimer level in a patent with a disorder associated with DIC, is called “nonovert, covert, or compensated DIC.” Compensated DIC is extremely difficult to diagnose and may be relatively common in certain underlying disorders where liver and bone marrow functions are normal. When it is suspected in such a situation, serial laboratory observation and aggressive treatment of the underlying disorder are usually important.
D-dimer levels are always increased in patients with DIC due to the consecutive action of thrombin, activated factor XIII, and plasmin, producing a neoepitope of fibrinogen that can be detected in whole blood by very sensitive immunoassays. Unfortunately, elevation of D-dimer is sensitive but nonspecific; D-dimer levels are increased in many other situations, including recent surgery, ascites, pleural effusion, soft-tissue bleeding, and inflammation. Thus, the various tests used to diagnose DIC require an understanding of the underlying disease; careful analysis of the platelet count, fibrinogen level, D-dimer level, and screening coagulation tests; and a review of the peripheral blood smear. The patient’s present clinical status and state of hepatic and bone marrow function must also be considered. Although the ISTH DIC scoring system provides an assessment at a given moment in time, serial measurements are very frequently helpful in establishing a definitive diagnosis.
Clinical Presentation, Type of Tumors, and Pathophysiology
In contrast to patients with subacute DIC, as seen in APL, or acute DIC, as seen in sepsis, patients with solid tumors often present without symptoms and only laboratory abnormalities that suggest the diagnosis of DIC. Other patients with solid tumors and DIC may present with excessive bleeding, venous or arterial thromboembolism, or nonbacterial endocarditis. An occasional patient may present with microangiopathic hemolytic anemia, thrombocytopenia, or both. The dominant tumor type associated with DIC is adenocarcinoma, and the tumors usually originate in the gastrointestinal tract (frequently signet ring cell type), pancreas, lung, breast, or prostate.[12] However, other types of tumors may occasionally be involved, including glioblastoma or other brain tumors.[33] The principal mechanism that triggers DIC in these patients is the generation of tissue factor by the tumor cells themselves, or increased tissue factor generated on the surface of monocytes or macrophages.[1,3,34-36] Tissue factor so generated then binds to factor VII, thereby activating factors IX and X. Moreover, increased tissue factor may be expressed by endothelial cells, which are also intimately involved in the regulation of blood coagulation because of the endothelium’s importance in protein C activation and the expression of tissue factor pathway inhibitor and thrombomodulin.[3] However, the degree to which these inhibitory pathways are affected in patients with solid tumors is not clear. In addition, some tumors generate a cysteine protease, which directly activates factor X.[1,3] Proinflammatory cytokines and tumor necrosis may further trigger procoagulant pathways, and chemotherapy may exacerbate this process.[3]
Treatment of Disseminated Intravascular Coagulation in Patients With Solid Tumors
Because of the tremendous heterogeneity of both the underlying disorders causing DIC and the clinical manifestations of DIC, treatment is controversial. The literature mostly consists of anecdotal reports and published recommendations achieved by consensus.[17-19,37-39] The only clinical trial of anticoagulant therapy was the one discussed above by Sakuragawa et al, where relatively low-dose LMWH or UFH given by continuous intravenous infusion appeared to stabilize or improve hemostatic abnormalities in most patients.[21] The principle of treatment in any patient with DIC is to try to eliminate the cause of the DIC. Thus, in solid tumors, if the patient can tolerate chemotherapy, then specific chemotherapy for the underlying tumor should be initiated. In addition, several important questions must be asked to determine whether replacement of depleted hemostatic factors is necessary and whether interruption of the DIC with heparin is to be considered. The suggested clinical steps associated with possible answers to the major questions outlined below reflect the published consensus recommendations by various societies, national committees, and the author’s experience and opinion.[17-19,37-43]
1(a) Does the patient have a low fibrinogen level, a low platelet count, a prolonged prothrombin time, or a combination of these? (b) If so, is the patient actively bleeding or at high risk for bleeding with no evidence of venous or arterial thromboembolism?
If the answer to the second question is no, and the patient does not require an invasive procedure to obtain tissue for pathologic examination, then replacement therapy is probably unnecessary. Exceptions would be if the platelet count is < 10,000/µL and there is a high risk of bleeding. In contrast, if the patient is actively bleeding or requires an invasive procedure for diagnosis and management of bleeding, or both, it probably will be necessary to attempt to replace the appropriate hemostatic factors, which usually means transfusion of cryoprecipitate, platelet concentrates, and, on occasion, fresh frozen plasma. When patients are euvolemic or hypervolemic, it is probably better to at least partially correct vitamin K–dependent clotting factor deficiencies with the newer unactivated concentrates rather than use large volumes of plasma. The argument that replacement therapy may “fuel the fire” and cause thrombosis is theoretically correct, but this has been shown to occur only occasionally. It is important to obtain a fibrinogen level and platelet count 45 to 60 minutes after completion of the transfusions to ascertain whether there has been a significant increase in the fibrinogen level and platelet count.
The expected increment in fibrinogen and platelets in the absence of a short half-life would be approximately 5 to 10 mg/dL per single unit of cryoprecipitate and approximately 5,000 to 10,000/µL per single unit of platelets (ie, 25,000 to 50,000/µL per apheresis unit). If an adequate increment is achieved and hemostasis is attained, then transfusions at appropriate intervals must be continued. If hemostasis is not achieved despite adequate correction of hemostatic factors, then the site of excessive bleeding should be sought by endoscopy, imaging, or both, and local hemostatic control attempted (Figure). Similarly, if the cause of the DIC is a suspected solid tumor, appropriate imaging and endoscopy must be performed, with tissue for pathologic evaluation obtained by the safest method deemed possible.
2. Does the patient have evidence of venous or arterial thromboembolism without excessive bleeding?
If the answer is yes, then it will be necessary to initiate anticoagulant therapy and maintain fibrinogen levels at ≥ 100 mg/L (approximately) and platelet counts > 50,000/µL via appropriate transfusions of cryoprecipitate and platelets. Continuous infusion of UFH should be started at a dose of 10 U/kg/h, with serial determinations of fibrinogen levels and platelet counts. If the platelet count (> 50,000/µL) and fibrinogen level (> 100 mg/dL) can be reasonably maintained, then the heparin dose can be incrementally increased by 100 to 200 U/h at 4-hour intervals over the next 24 hours. The activated partial thromboplastin time should also be followed and should be prolonged no more than 1.5 to 2 times the normal time. The patient should be carefully observed for evidence of bleeding (venipuncture sites, stool examination, urine color, headache, and decreasing hemoglobin level). If the cause of DIC is unknown but a solid tumor is suspected and the patient requires an invasive diagnostic procedure, then the UFH can be temporarily interrupted and tissue obtained for pathologic examination (see Figure).
On occasion, a patient with DIC presents with severe bleeding and venous thrombosis of one or both lower extremities. In this situation, anticoagulation is contraindicated, and an inferior vena cava filter must be placed. In addition, low hemostatic factors must be increased, local control of bleeding achieved, and anticoagulant therapy initiated when relatively safe (see Figure).
Although antithrombin III, activated protein C, thrombomodulin, and tissue factor pathway inhibitor have been used to treat DIC associated with sepsis, there has been no published experience with these concentrates in DIC associated with malignant disease.
3. When can chemotherapy be initiated?
If the origin and type of tumor are known, then specific chemotherapy should be initiated as soon as the clinical problems associated with DIC are under control. If not known, then the type of tumor must be sought per clinical clues, appropriate imaging, and possibly endoscopy, and pathologic tissue must be obtained by biopsy, fine needle aspiration, or brushing and cytology. If there is no evidence of excessive bleeding and no evidence of thromboembolic complications, then strong consideration should also be given to initiating anticoagulant therapy because the chemotherapy may exacerbate the DIC and result in thrombotic and/or bleeding problems (see Figure). Unfortunately, these complications are unpredictable, and so the decision to initiate anticoagulant therapy is a difficult one. If the decision is made to start anticoagulant therapy, intravenous UFH should be started at a dose of 10 U/kg/h. Platelet counts and fibrinogen levels should be monitored to assess whether these are stable or increasing to relatively safe levels.
Although in the study by Sakuragawa et al, the results were insignificantly better with LMWH than with UFH, the short half-life and reversibility of UFH favor its use in this particular situation. When the patient’s hemostatic status is stabilized and there are no comorbidities that would prohibit the initiation of chemotherapy, treatment can be started.
After chemotherapy is initiated, continuous adjustments must be made to balance the amount of heparin against the platelet count and fibrinogen level. Once the latter are stabilized or increasing and there is no evidence of thromboembolic or bleeding complications, then consideration should be given to substituting LMWH for UFH in prophylactic-to-intermediate doses. Interestingly, although there are only a few anecdotal case reports, if the patient has an initial response to chemotherapy, the DIC appears to progressively improve (increasing platelet counts and fibrinogen levels) in the first 2 weeks of treatment.[23-25,28,43,44]
Conclusion
This review and these treatment recommendations reflect the lack of solid data and the extremely complex management of patients with overt DIC associated with solid tumors. Patients with so-called covert DIC were not discussed, because unequivocal proof of overt DIC is lacking and most of these patients are usually given prophylactic heparin when they are given chemotherapy. In addition, many of these patients may present with thromboembolic complications and so receive therapeutic doses of heparin throughout their treatment course. Although the management of DIC in patients with solid tumors is complicated, aggressive supportive hemostatic replacement therapy, careful use of anticoagulants, or both, may provide a window of opportunity to give effective chemotherapy and obtain a meaningful remission, depending on the type of underlying tumor. Thus, if this serious complication is managed urgently and aggressively, a meaningful response and prolonged survival can be achieved.
Financial Disclosure:The author has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Levi M, Feinstein DI, Colman RW, Marder VJ. Consumptive thrombohemorrhagic disorders. In: Marder VJ, Aird WC, Bennett J, et al, editors. Hemostasis and thrombosis, basic principles and clinical practice. 6th ed. Philadelphia: Wolters Kluwer/Lippincott, Williams & Wilkins; 2012. p.1178-95.
2. Sarris AH, Kempin S, Berman E, et al. High incidence of disseminated intravascular coagulation during remission introduction of adult patients with acute lymphoblastic leukemia. Blood. 1992;79:1305-10.
3. Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol. 2009;22:129-36.
4. Kwann H, Barnett M, Cull EH. The coagulopathy in acute promyelocytic leukaemia-what have we learned in the past twenty years. Best Pract Res Clin Haematol. 2014;27:11-18.
5. Breccia M, Lo Coco F. Thrombo-hemorrhagic deaths in acute promyelocytic leukemia. Thromb Res. 2014;133:S112-6.
6. Rashidi A, Silverberg ML, Conkling PR, et al. Thrombosis in acute promyelocytic leukemia. Thromb Res. 2013;131:281-9.
7. Chang H, Kuo MC, Shih LY, et al. Acute promyelocytic leukemia-associated thrombosis. Acta Haematol. 2013;130:1-6.
8. Mitrovic M, Suvajdzic N, Bogdanovic A, et al. International Society of Thrombosis and Hemostasis Scoring System for disseminated intravascular coagulation ≥6: a new predictor of hemorrhagic early death in acute promyelocytic leukemia. Med Oncol. 2013;30:478.
9. Chodhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. Am J Hematol. 2012;87:596-603.
10. Trousseau A. Phlegmasia alba dolens. Clinique Medicale de l’Hotel-Dieu de Paris. Paris Bailliere. 1865;3:654-712.
11. Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine. 1977;56:1-37.
12. Sallah S, Wan JY, Hguyen NP, et al. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828-33.
13. Taylor FB Jr, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation-on behalf of the Scientific Subcommittee on DIC of the ISTH. Thromb Haemost. 2001;86:1327-30.
14. Bakhtiari K, Meijers J DeJonge E, et al. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2416-21.
15. Sivula M, Tallgren M, Pettila V. Modified score for disseminated intravascular coagulation in the critically ill. Intensive Care Med. 2005;31:1209-14.
16. Toh CH, Hoots WK. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5:604-6.
17. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145:24-33.
18. Di Nisio M, Baudo F, Cosmi B, et al. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2012;129:e177-84.
19. Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11:761-7.
20. Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2:2-15.
21. Sakuragawa N, Hasegawa H, Maki M, et al. Clinical evaluation of low-molecular-weight heparin (FR-860) on disseminated intravascular coagulation (DIC)-a multicenter co-operative double blind trial in comparison with heparin. Thromb Res. 1993;72:475-500.
22. Aoki N, Hasegawa H. About revision the item entitled “Auxiliary clinical results and findings for diagnosis” in the DIC Diagnostic Criteria. Ministry of Health and Welfare Research Committee on Specific Diseases, Blood Coagulation Abnormalities; research report for fiscal year 1987.1988;37-41.
23. Hwang IG, Choi JH, Park SH, et al. Chemotherapy in advanced gastric cancer patients associated with disseminated intravascular coagulation. Cancer Res Treat. 2014;46:27-32.
24. Yeh KH, Cheng AL. Gastric cancer associated with acute disseminated intravascular coagulation: successful initial treatment with weekly 24-hour infusion of high-dose 5-fluorourarcil and leucovorin. Br J Haematol. 1998:100:769-72.
25. Chao Y, Teng HC, Hung HC, et al. Successful initial treatment with weekly etoposide, epirubicin, cisplatin, 5-fluororouracil and leucovorin chemotherapy in advanced gastric cancer patients with disseminated intravascular coagulation. Jpn J Clin Oncol. 2000;30:122-5.
26. Tokar M, Bobilev D, Ariad S, Geffen DB. Disseminated intravascular coagulation at presentation of advanced gastric cancer. Isr Med Assoc J. 2006;8:853-5.
27. Huang TC, Yeh KH, Cheng AL, et al. Weekly 24-hour infusional 5-fluororacil as initial treatment for advanced gastric cancer with acute disseminated intravascular coagulation. Anticancer Res. 2008:28:1293-7.
28. Rhee J, Han SW, Oh DY, et al. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010;25:1537-42.
29. Takashima A, Shirao K, Hirashima Y, et al. Sequential chemotherapy with methotrexate and 5-fluorouracil for chemotherapy-naive advanced gastric cancer with disseminated intravascular coagulation at initial diagnosis. J Cancer Res Clin Oncol. 2010;136:243-8.
30. Klenner AF, Greinacher A, Kuvikova A, et al. Severe disseminated coagulopathy caused by adenocarcinoma with bone marrow metastasis. Onkologie. 2013;36:292-94.
31. Van Bunderen CC, de Weger VA, Griffioen-Keijzer A. Disseminated intravascular coagulation as clinical manifestation of colorectal cancer: a case report and review of the literature. Neth J Med. 2014;72:186-89.
32. Levi M, Meijers JC. DIC: which laboratory tests are most useful. Blood Rev. 2011;25:33-7.
33. Cai J, Zhang Y, Bai X, et al. Postoperative hemorrhage in an elderly patient with a glioblastoma multiform and a calcified chronic subdural hematoma. World J Surg Oncol. 2014;12:110.
34. Rickles FR, Brenner B. Tissue factor and cancer. Semin Thromb Hemost. 2008;34:143-5.
35. Zwicker JI, Liebman HA, Neuberg D, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830-40.
36. Falanga A, Lee AYY, Rickles FR. Cancer related thrombosis. In: Marder VJ, Aird WC, Bennett J, et al, editors. Hemostasis and thrombosis, basic principles and clinical practice. 6th ed. Philadelphia: Wolters Kluwer/Lippincott, Williams & Wilkins; 2012. p. 1468-80.
37. Wada H, Asakura H, Kohji O, et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125:6-11.
38. Thachil J, Toh CH. Current concepts in the management of disseminated intravascular coagulation. Thromb Res. 2012;129:s54-9.
39. Levi M. Japanese consensus for disseminated intravascular coagulation (DIC): Is it a small world after all? Thromb Res. 2010;125:4-5.
40. Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-7.
41. Feinstein DI. Disseminated intravascular coagulation: how to intervene in a complex process. J Crit Illness. 1989;4:21-39.
42. Feinstein DI. Treatment of disseminated intravascular coagulation. Semin Thromb Hemost. 1988;14(4):351-62.
43. Mast C, Ramanathan RK, Feinstein DI, Rosen P. Disseminated intravascular coagulation secondary to advanced pancreatic cancer treated successfully with combination chemotherapy. Oncology. 2014;87:266-9.
44. Ferrand FR, Gontier E, Guymar S, et al. Effectiveness and safe use of modified FOLFOX-6 for metastatic gastric cancer with signet ring cell components complicated by disseminated intravascular coagulation and diffuse bone marrow carcinomatosis. Onkologie. 2012;35:118-20.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.