Early Breast and Prostate Cancer and Clinical Outcomes (Fracture)
Over 40 million men and women in the United States have osteoporosis and low bone mineral density (BMD), placing them at risk for adverse skeletal events such as fractures and their sequelae. There are over 12 million cancer survivors in this country. Of these, 22% were diagnosed with breast cancer and 17% with prostate cancer.[1,2] Because cancer therapies can adversely influence bone health, these survivors are at particular risk for skeletal complications. Cancer therapies associated with bone loss include hormone deprivation therapies such as aromatase inhibitors, ablative surgical procedures that induce hypogonadal states, and premature menopause induced by chemotherapy.[3,4]
Osteoporosis is a skeletal disorder characterized by low bone mass that is associated with increased risk of fracture. Nearly 40% of the 12 million cancer survivors in the United States were diagnosed with breast and prostate cancer. Therapy for these two diseases is not uncommonly associated with bone loss related to hormone-ablative therapy. In women, this includes the use of endocrine therapies and chemotherapy-related premature menopause. In men, hormone-ablative therapies include gonadotropin-releasing hormone analogs and bilateral orchiectomy. Fracture risk assessment includes bone mineral density determination in appropriate populations and integration of findings with identified risk factors. Strategies to prevent and treat bone loss include nonpharmacologic and pharmacologic interventions. In the former case, regular weight-bearing and muscle-strengthening exercise is encouraged along with smoking cessation, modulation of alcohol consumption, and fall prevention. Supplementation with calcium and vitamin D decreases fracture risk in subgroups. Pharmacologic interventions include use of oral or intravenous bisphosphonates, selective estrogen receptor modulators, and calcitonin. Estrogen/menopause hormone therapies are not recommended for use in breast cancer survivors related to potential influence on recurrence. Strategies for management of bone loss in breast and prostate cancer are outlined by guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network.
Over 40 million men and women in the United States have osteoporosis and low bone mineral density (BMD), placing them at risk for adverse skeletal events such as fractures and their sequelae. There are over 12 million cancer survivors in this country. Of these, 22% were diagnosed with breast cancer and 17% with prostate cancer.[1,2] Because cancer therapies can adversely influence bone health, these survivors are at particular risk for skeletal complications. Cancer therapies associated with bone loss include hormone deprivation therapies such as aromatase inhibitors, ablative surgical procedures that induce hypogonadal states, and premature menopause induced by chemotherapy.[3,4]
Bone Biology
Healthy bone is maintained by a balance between osteoclasts that break down bone and osteoblasts that form new bone. In normal bone, the cycle of resorption and formation is balanced and regulated by complex mechanisms involving systemic and local factors. As one factor, estrogen decreases the formation, activation, and lifespan of osteoclasts. Loss of estrogen from menopause or from iatrogenic causes results in a remodeling imbalance with a prolonged osteoclast-induced resorption phase and a reduced formation phase.[3,4] In addition, loss of androgens also leads to increased bone resorption through decreased aromatization of androgens to estrogens in bone.[5]
Factors involved in the control of bone remodeling include rRANK ligand and osteoprotegerin (OPG).[6] RANK ligand is expressed by osteoblasts and, as a small molecular signal, activates and binds to the RANK receptor on osteoclasts promoting both osteoclast formation and prolongation of the osteoclast lifespan. Osteoprotegerin suppresses the effects of RANK ligand by posing as a RANK ligand decoy receptor, thus reducing RANK receptor binding. Change in the ratio between RANK ligand and OPG balance contributes to bone disease. The currently approved pharmacologic agents used to treat bone loss, bisphosphonates, work by inhibiting osteoclast-mediated bone resorption.
Bone Health in Breast Cancer Survivors
Cancer therapy may be associated with bone loss. The most common cause is the use of hormone-ablative therapy, including endocrine therapies and chemotherapy-related premature menopause in women. In men, hormone-ablative therapies include use of gonadotropin-releasing hormone (GnRH) analogs and bilateral orchiectomy.
Endocrine Therapy
The majority of patients with breast cancer have hormone receptor–positive disease and receive adjuvant therapy with either a selective estrogen receptor modulator (SERM) and/or an aromatase inhibitor. In postmenopausal women, SERM reduces bone resorption and fracture risk. In contrast, the aromatase inhibitors, which block conversion of androgens to estrogen, increase bone resorption by prolonging osteoclast-induced resorption. The aromatase inhibitors are increasingly used in the adjuvant setting of hormone receptor–positive breast cancer in postmenopausal women as they significantly improve disease-free survival compared to SERMs and have less risk of venous vascular events.[7] However, aromatase inhibitors are associated with loss of BMD and increased fracture risk.
FIGURE 1
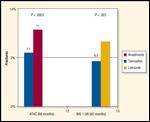
Fracture Rates
In substudies of the larger adjuvant breast cancer trials involving postmenopausal women with early-stage breast cancer, higher rates of bone loss have been seen in women receiving aromatase inhibitors compared to women on placebo or SERM. A prospective bone substudy in the ATAC trial (Anastrozole, Tamoxifen, Alone or in Combination) found that, after 5 years, mean BMD decreased by 6.1% in the spine and 7.2% in the hip for patients on the aromatase inhibitor, but increased by 2.8% in the spine and 0.7% in the hip for those receiving the SERM. No women with an initial normal bone density at the start of treatment developed osteoporosis after 5 years of treatment. Those whose baseline T score was less than -1.5 prior to initiation of the aromatase inhibitor were at greatest risk for developing osteoporosis.[8] In similar trials with two other aromatase inhibitors bone loss was consistently higher with the aromatase inhibitor.[9,10] It has become clear that the aromatase inhibitors cause bone loss for the duration of their administration regardless of which aromatase inhibitor is selected. The bone loss seen with aromatase inhibitor use is associated with increased fracture risk;[11,12] without intervening therapy there is about a 10% risk of clinical fracture after 5 years of aromatase inhibitor use (Figure 1).
Adjuvant Chemotherapy
The majority of the more than 2 million breast cancer survivors in the United States are either postmenopausal at diagnosis or they become menopausal as a result of cytotoxic chemotherapy. Loss of BMD is observed in women undergoing adjuvant chemotherapy due to both treatment-induced premature ovarian failure and possibly direct effects of chemotherapy on bone.
Premenopausal women with early-stage breast cancer who become amenorrheic experience accelerated bone loss of about 6% to 8% in the first year. The loss of bone persists for years after completion of chemotherapy in women who continue to be amenorrheic[13] primarily related to loss of ovarian function.[14]
Prostate Cancer and Bone Health
Men with early-stage prostate cancer are more likely to have low BMD than age-matched controls without prostate cancer.[15] In addition, treatment of prostate cancer with androgen deprivation therapy using a GnRH analog, with or without an antiandrogen, reduces estrogen and testosterone levels with associated increase in bone resorption and decrease in bone formation.[16]
In cross-sectional studies in men diagnosed with nonmetastatic prostate cancer, those treated with GnRH agonists experience significant bone loss after 1 year of about 6% loss in BMD compared to men with prostate cancer who are not receiving systemic therapy.[17] In one study, 5 years after diagnosis, 19.6% of men treated with androgen deprivation therapy sustained fracture compared to 12.6% of men not receiving that treatment (P < .001).[18] The severity of the reduction in BMD correlates with the duration of androgen deprivation therapy. Therapy with a GnRH analog for 4 or more years was associated with a greater fracture risk compared with treatment for 1 year or less (hazard ratio [HR] 1.5 [P < .001]).[19]
Evaluation of Bone Health
The 2003 American Society of Clinical Oncology (ASCO) guidelines published by Hilner and colleagues presented an algorithm for maintenance of bone health in women with breast cancer.[20] Women are at high risk for osteoporosis if they are older than 65, or between the ages of 60 and 65 with risk factors including family history of fracture, body weight less than 70 kg, prior nontraumatic fracture, Asian or White ethnicity, smoking, or having risk factors for falls or an inactive lifestyle. Postmenopausal women treated with aromatase inhibitors and premenopausal women with therapy-related premature menopause are also considered at increased risk. Accordingly, individuals at high risk should undergo DEXA screening with results subsequently guiding therapy.
In both men and women with cancer secondary causes of poor skeletal health have been identified. In addition to age-related bone loss, lifestyle factors such as smoking, exercise habits, poor vitamin D and calcium intake, and alcohol consumption may contribute to poor bone health. Other medical conditions including hypogonadism, growth hormone deficiency, hyperthyroidism, hyperparathyroid issues, hypercalciuria, and glucocorticoid excess can contribute to bone loss.[17] Iatrogenic causes of bone loss in addition to hormone-ablative therapy include prolonged use of an anticoagulant, glucocorticoids, anticonvulsants, and antimetabolite/antifolate agents.
Evaluation for Fracture Risk
Previously, several organizations including the World Health Organization (WHO), the National Osteoporosis Foundation (NOF), and the North American Menopause Society have recommended pharmacologic therapy for those with osteoporosis (T score on BMD of less than -2.5) or those with a prior fragility fractures (hip or vertebral fracture). More recently, as interest in more comprehensive models for fracture has emerged,[21,22] it has become possible to more accurately predict fracture risk. In this regard, the WHO has developed a web-based Fracture Risk Assessment Tool (FRAX) that integrates clinical risk factors as well as BMD information. Model components include age, sex, weight, height, previous fracture, parent with hip fracture, smoking, glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol (> 3 units/d), and femoral neck BMD (Figure 2).
Using the web-based FRAX model or simplified paper versions, readily available at the FRAX website (http://www.shef.ac.uk/FRAX/), the 10-year probability of hip fracture and the 10-year probability of a major osteoporosis fracture (clinical spine, forearm, hip, or shoulder fracture) can be calculated.[23] Treatment decisions generally follow country-specific guidelines. The US-based NOF recommends pharmacologic interventions when the BMD T score is between -1.0 and -2.5) and the 10-year probability of hip fracture is ≥ 3% or major osteoporosis-related fracture is > 20%.
At this time, the effect of hormone-ablative therapy is not incorporated in the FRAX model. The rate of bone loss with aromatase inhibitors is greater than that seen with smoking but less than that associated with corticosteroid use. In any event, the clinical fracture risk of about 10% after 5 years of aromatase inhibitor use (Figure 1) places women at substantial fracture risk supporting pharmacologic intervention.
Prevention and Management of Bone Loss
TABLE 1
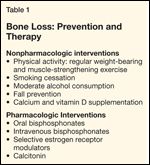
Bone Loss: Prevention and Therapy
Prevention and management of bone loss involves both lifestyle modification and pharmacologic therapy (Table 1). Lifestyle modifications focus on weight-bearing and muscle-strengthening exercise, smoking cessation, alcohol moderation/avoidance, and fall prevention.
Calcium and Vitamin D
While calcium and vitamin D supplementation represent an integral component of bone health guidelines and vitamin D deficiency is a recognized secondary cause of bone health, there is controversy regarding optimal monitoring and intervention approaches with respect to vitamin D. A full discussion of this issue is beyond the scope of this report.
The current evidence in this area has been summarized in a recent Cochrane database systematic review which concluded that, based on randomized controlled trials, vitamin D (dose 400–800 IU/D) when given with calcium supplementation reduces risk of nonvertebral and hip fractures, perhaps mainly in subgroups.[24] In the Women’s Health Initiative (WHI) randomized clinical trial evaluating supplementation with calcium and vitamin D3 (400 IU/d), an increase in BMD of about 1% was seen.[25] In addition, a meta-analysis of randomized controlled trials evaluating vitamin D (with the WHI trial a major contributor) where the mean daily dose in the trials was 528 IU identified a significant reduction in total mortality with vitamin D use (HR 0.92; 95% confidence interval 0.86–0.99).[26] Thus, routine supplementation in appropriate populations can be recommended. Postmenopausal women and older men need about 1,500 mg of calcium daily. Vitamin D at a dose 800 to 1,000 IU a day is also recommended.
While some recommend monitoring 25-hydroxyvitamin D levels and aggressive vitamin D supplementation with parenterally administered high-dose vitamin D regimens, such an approach can be questioned given the limited clinical trial evidence for such a strategy. A recent systematic review found “inconsistent evidence” of an association between lower 25-hydroxyvitamin D levels and increased fracture risk and went on to state that it is “difficult to define optimal 25-hydroxyvitamin D levels for bone health.”[27] In addition, only a modest component of person-to-person variability in 25-hydroxyvitamin D levels can be explained by differences in dietary and supplement vitamin D intake and/or sunlight exposure.[28]
Health-care providers should follow developments in this area as studies beginning with individuals with low 25-hydroxyvitamin D levels, provision of supplementation sufficient to increase vitamin levels to a prospective target, and subsequent impact on clinical endpoints such as fracture risk are currently lacking and represent a research priority.
Pharmacologic Interventions
Bisphosphonates are clinically utilized to preserve BMD as they inhibit osteoclast-mediated bone resorption through the structural characteristic phosphonate-carbon-phosphonate which promotes binding to bone mineral matrix. Clinical studies have demonstrated that bisphosphonates increase BMD and can largely abrogate the bone loss associated with hormone-ablative therapy in women.[29,30] Multiple prospective studies involving postmenopausal women with osteoporosis treated with bisphosphonates have shown significant reductions in vertebral and nonvertebral fracture risk.[31,32] However, there have been no studies specifically designed to evaluate the reduction in osteoporotic fracture risk among cancer survivors. Therefore, the aforementioned recommendations for bisphosphonate use for bone loss prevention in cancer survivors are based on studies showing prevention of osteoporotic fractures in a healthy population.[33] In terms of side effects, patient adherence to long-term oral bisphosphonate regimens has proven problematic. Osteonecrosis of the jaw can occur but is rarely encountered with bisphosphonates dosed for bone health. Intravenous bisphosphonates can be associated with infusion-related bone pain and myalgias.
Use of menopausal hormone therapy is not recommended to treat bone loss in breast cancer survivors, given the potential influence on recurrence risk.[34] Similarly the use of a bone anabolic agent is generally avoided in cancer survivors given the theoretical risk of bone cancer seen in animal models.[20,36]
Guidelines
The ASCO guidelines for bone health maintenance in women with diagnosed breast cancer recommend treatment based on DEXA score:[21] For T scores of -2.5 or lower (osteoporotic range), increased physical activity, calcium and vitamin D supplementation, and treatment with a bisphosphonate is recommended. For osteopenia (T score is between -1 and -2.5) or normal bone health (T score > -1), lifestyle modification and calcium with vitamin D supplementation are recommended and bisphosphonate therapy can be considered. For those not at high risk for osteoporosis it is also recommended that they adhere to appropriate lifestyle modifications and initiate calcium and vitamin D supplementation but BMD screening is not recommended. In their current practice guidelines, the National Comprehensive Cancer Network recommends BMD monitoring for premenopausal women with ovarian failure secondary to adjuvant chemotherapy and postmenopausal women treated with an aromatase inhibitor.[36]
The management of bone loss in men with prostate cancer is evolving. The recommendations for lifestyle modification and calcium and vitamin D supplementation apply to older men as well as women. The data support assessment of BMD with a DEXA scan in all men with newly diagnosed prostate cancer. Treatment with a bisphosphonate is prudent for those who are osteoporotic. If the BMD is between -1.5 and -2.5, the decision for treatment should be based on the patient’s comorbidities and fall risk.
Conclusions
One of the major factors contributing to bone health compromise in patients with prostate and breast cancer is hormone-ablative therapy resulting in reduction in androgen and estrogen levels. Moreover, the natural course of postmenopausal development of osteoporosis, as well as other secondary causes of osteoporosis, may also contribute to bone loss in the cancer setting. All patients undergoing treatment for breast cancer and prostate cancer need evaluation of their state of bone health and fracture risk. If there is evidence of osteopenia or osteoporosis at baseline, secondary causes of bone loss should be excluded and a discussion of lifestyle modification factors, supplementation with calcium and vitamin D, and if appropriate, pharmacologic treatment needs to be initiated. Ongoing studies are evaluating newer agents for potential use in this setting.
Financial Disclosure:Dr. Chlebowski has received speaker’s fees and honoraria for advisory boards and consulting from AstraZeneca and Novartis; honoraria for advisory boards and consulting for Lilly, Amgen, and Pfizer; and grant support from Amgen, NIH, and the National Cancer Institute of Canada.
This article was conceived of and fully funded by Amgen, and Amgen provided background direction for the article.
References:
1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785-795, 2001.
2. Mazzuoli G, Marinucci D, D’erasmo E, et al: Cyclical behavior of bone remodeling and bone loss in healthy women after menopause: results of a prospective study. Bone 31(6):718-724, 2002.
3. Akhtari M, Mansuri J, Newman KA, et al: Biology of breast cancer bone metastasis. Cancer Biol Ther 7(1):3-9, 2008.
4. Roodman GD: Mechanisms of bone metastasis. N Engl J Med 350(16):1655-1664, 2004.
5. Dougall WC, Glaccum M, Charrier K: RANK is essential for osteoclast and lymph node development. Genes Devel 13:2412-2424, 1999.
6. Blair JM, Zhou H, Seibel MJ, Dunstan CR: Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol 3(1):41-49, 2006.
7. Burkiewicz JS, Scarpace SL, Bruce SP: Denosumab in osteoporosis and oncology. Ann Pharmacother 43(9):1445-1455, 2009.
8. Jones DH, Naskashima T, Sanchez OH, et al: Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440(7084):692-696, 2006.
9. Canon JR, Roudier M, Bryant R, et al: Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 25(2):119-129, 2008.
10. Gralow JR, Biermann JS, Farooki A, et al: NCCN Task Force report: bone health in cancer care. J Natl Compr Canc Netw 7(suppl 3):S1-S32, 2009.
11. Ramaswamy B, Shapiro CL: Osteopenia and osteoporosis in women with breast cancer. Semin Oncol 30(6):763-775, 2003.
12. Shevde NK, Bendixen AC, Dienger KM, Pike JW: Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A 97(14):7829-7834, 2000.
13. Bell NH: RANK ligand and the regulation of skeletal remodeling. J Clin Invest 111(8):1120-1122, 2003.
14. Shapiro CL: Aromatase inhibitors and bone loss: risks in perspective. J Clin Oncol, 23(22):4847-4849, 2005.
15. Raisz LG: Physiology and pathophysiology of bone remodeling. Clin Chem 45(8 pt 2):1353-1358, 1999.
16. Guise TA: Bone loss and fracture risk associated with cancer therapy. Oncologist 11(10):1121-1131, 2006.
17. Lonning P: Endocrine therapy and bone loss in breast cancer: time to close in the RANK(L)? J Clin Oncol 26(30):4859-4861, 2008.
18. Perez EA, Weilbaecher K: Aromatase inhibitors and bone loss. Oncology (Williston Park), 20(9):1029-1039; discussion 1039-1040, 1042, 1048, 2006.
19. Hillner BE, Ingle JN, Chlebowski RT, et al: American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21(21):4042-4057, 2003. Erratum in: J Clin Oncol 22(7):1351, 2004. Dosage error in article text.
19A. Hadji P, Body JJ, Aapro MS, et al: Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol 19(8):1407--1416, 2008.
20. Israeli RS, Ryan CW, Jung LL: Managing bone loss in men with locally advanced prostate cancer receiving androgen deprivation therapy. J Urol 179(2):414-423, 2008.
21. Oefelein MG, Ricchiuti V, Conrad W, Resnick MI: Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 168(3):1005-1007, 2002.
22. Satoh T, Kimura M, Matsumoto K, Tabata K, et al: Single infusion of zoledronic acid to prevent androgen deprivation therapy-induced bone loss in men with hormone-naive prostate carcinoma. Cancer 115(15):3468-3474, 2009.
23. Esteve FR, Roodman GD: Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol 20(4):613-624, 2007.
24. Heath DJ, Vanderkerken K, Cheng X, et al: An osteoprotegerin-like peptidomimetic inhibits osteoclastic bone resorption and osteolytic bone disease in myeloma. Cancer Res 67(1):202-208, 2007.
25. Berenson JR, Rajdev L, Broder M: Bone complications in multiple myeloma. Cancer Biol Ther 5(9):1082-1085, 2006.
26. Roodman GD: Pathogenesis of myeloma bone disease. Leukemia 23(3):435-441, 2009.