Emergency Fertility Preservation in a Young Woman With Non-Hodgkin Lymphoma
A nulliparous woman, age 25 years, had received a diagnosis of non-Hodgkin lymphoma and now presented with stage IIA diffuse large B-cell lymphoma. Due to potential risks of chemotherapy-induced gonadotoxicity and subsequent iatrogenic premature ovarian failure and fertility loss, the patient was referred to the reproductive medicine department for fertility preservation counseling and further management.
Oncology (Williston Park). 2021;35(6):332-334.
DOI: 10.46883/ONC.2021.3506.0332
Salama is director of the Oncofertility Consortium in the Department of Obstetrics, Gynecology and Reproductive Biology, at the College of Human Medicine of Michigan State University in East Lansing.

Isachenko is head of the In Vitro Fertilization (IVF) Laboratory in the Department of Obstetrics & Gynecology at the University of Cologne in Germany.

Rahimi is head of Gynecological Endocrinology and Reproductive Medicine in the Department of Obstetrics & Gynecology at the University of Cologne in Germany.

Isachenko is head of the Research Group for Reproductive Medicine and IVF Laboratory in the Department of Obstetrics & Gynecology at the University of Cologne in Germany.

Mallmann is director and head of the Department of Obstetrics & Gynecology at the University of Cologne in Germany.

The Case
A nulliparous woman, age 25 years, had received a diagnosis of non-Hodgkin lymphoma (NHL) and now presented with stage IIA diffuse large B-cell lymphoma (DLBCL). According to her hematological oncologist’s treatment plan, chemotherapy had to start immediately (within 1 week), with the patient receiving 6 courses of the standard R-CHOEP21 regimen (rituximab 375 mg/m², cyclophosphamide 750 mg/m², hydroxydaunorubicin 50 mg/m², vincristine 1.4 mg/m², etoposide 100 mg/m², prednisone 40 mg/m²).
Due to potential risks of chemotherapy-induced gonadotoxicity and subsequent iatrogenic premature ovarian failure (POF) and fertility loss, the patient was referred to the reproductive medicine department for fertility preservation counseling and further management.
With the aforementioned fi ndings, what should be recommended for fertility preservation in this patient?
Discussion
NHL is the most common type of lymphoma, representing 88% of lymphoma cases, and typically occurs in adolescents and young adults more than in children.1-3 In this patient, several challenges are raised regarding fertility preservation, including that:
(1) the patient is a young adult who wants to have children in the future. Overall survival (OS) in NHL has increased dramatically in recent years, especially in young adults (5-year OS, 69%; 10-year OS, 64%), giving the patient reason to look forward to her survivorship and possible parenthood. Still, OS is not 100%;
(2) the chemotherapy regimen R-CHOEP21 includes the alkylating agent cyclophosphamide. Cumulative doses of aggressive alkylating agents can lead to gonadotoxicity and subsequent iatrogenic POF and fertility loss in most cases;
(3) if the patient’s disease becomes relapsed or refractory, hematopoietic stem cell transplant (HSCT) may be indicated, in which case pre-HSCT myeloablative conditioning regimens will be used to induce immunosuppression. These regimens, including total body irradiation (TBI) and/or high-dose alkylating chemotherapy, lead to severe gonadotoxicity and subsequent iatrogenic POF and fertility loss in almost 100% of cases;
(4) DLBCL, the most aggressive (and most common) form of NHL, requires immediate chemotherapy initiation, leaving not enough time to apply several fertility preservation options;
(5) the traditional options of embryo cryopreservation and oocyte cryopreservation (answer choices A & B) need prior ovarian stimulation for some weeks to retrieve mature oocytes that are ready for in vitro fertilization, and that is not feasible in this patient due to lack of time;
(6) the innovative option of ovarian tissue cryopreservation and further autotransplantation (answer choice C) is suitable for emergency fertility preservation, but it is also risky due to possible contamination of ovarian tissue with lymphoma cells; and
(7) ovarian protection options (answer choice D), which include oophoropexy, pelvic shielding, and gonadotropin-releasing hormone (GnRH) analogues, are not reliable. Oophoropexy and pelvic shielding do not protect ovaries when systemic chemotherapy is used, while GnRH analogues have not been proven to protect the ovaries in patients with lymphoma, and they do not protect ovaries if TBI is used.1-3
Management
To overcome the aforementioned challenges, we discussed options with the patient and decided that ovarian tissue extraction and cryopreservation (answer choice C) was the most suitable emergency fertility preservation option.4 The first step involved routine ovarian function tests; results were satisfactory and informed consent was obtained from the patient prior to the surgical procedure.
After coordinating with her hematological oncologists, the patient was admitted to our department and her right ovary was excised via laparoscopy 3 days before chemotherapy initiation. The excised ovarian tissue was transported to our laboratory within 10 minutes of extraction for further processing. As previously described by our group,5 the basal medium used for transport and dissection was composed of Leibovitz L-15 Medium supplemented with 5% dextran serum substitute. The temperature of the sample was maintained between 32 °C and 34 °C. Afterward, ovarian cortex was dissected into small medulla-containing strips, each measuring 0.5 to 1 cm square and 1 to 2 mm thick.
The ovarian tissue strips were then cooled at 5 °C for 24 hours, followed by cryopreservation using a slow freezing protocol. Cryopreservation began with placing the ovarian tissue strips for 30 minutes at room temperature in 20 mL of freezing medium, composed of basal medium supplemented with 6% dimethyl sulfoxide, 6% ethylene glycol, and 0.15 M sucrose. Then, each strip was placed into a standard 5-mL cryovial previously filled with 4.5 mL freezing medium and frozen in an 14S freezer. The slow cooling profile began at –6 °C, then the cryovials were cooled from −6 °C to −34 °C at a rate of −0.3 °C/min. This slow-freezing protocol included an autoseeding step at −6 °C. Finally, at −34 °C, the cryovials were plunged into liquid nitrogen (−196 °C) and stored for future use.
Prognosis
The patient plans to complete her anticancer therapy and become fit in recovery. When she would like to become pregnant, a new assessment of her endocrine and reproductive ovarian functions will be performed. If it is found that she has POF induced by the anticancer therapy, she can then use her cryopreserved ovarian tissue to attempt to restore her fertility. The success rate of ovarian tissue cryopreservation and further autotransplantation has been reported recently at around a 25% live birth rate per transplant.6 This success rate is promising but not conclusive; it seems to be case dependent and should be extrapolated with caution to the patient during counseling.
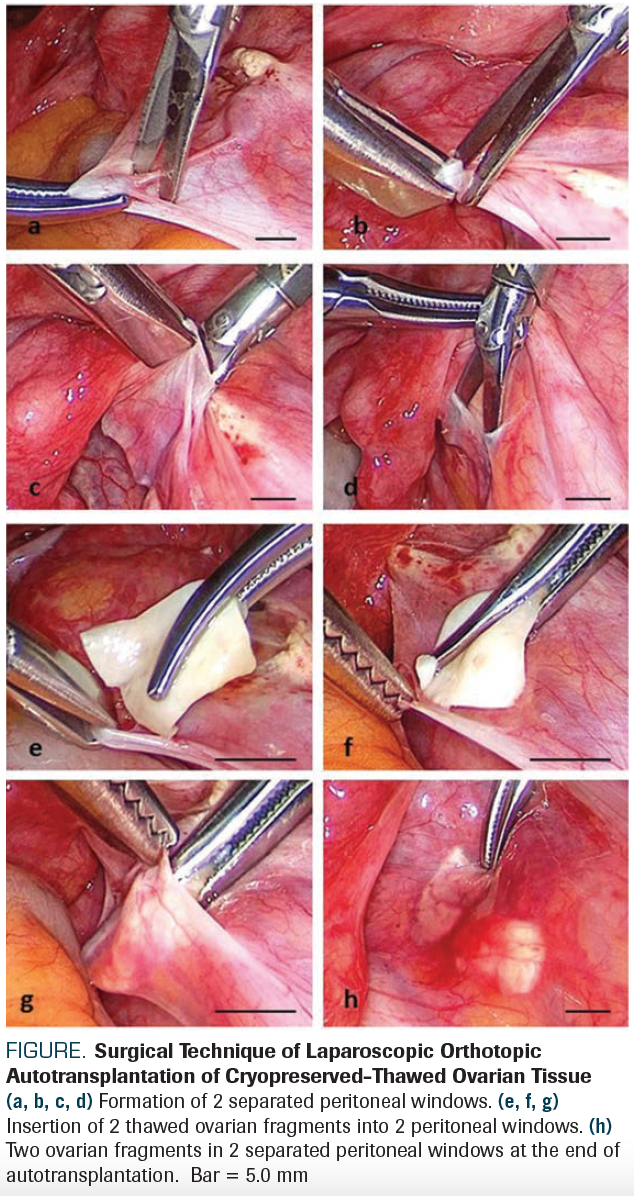
Technically, autotransplantation of cryopreserved-thawed ovarian tissue can be performed orthotopically into the remaining ovary, ovarian fossa, or broad ligament, or heterotopically into the subcutaneous space of the anterior abdominal wall or forearm. So far, the most successful method seems to be laparoscopic orthotopic autotransplantation of cortical ovarian tissue that was slowly cryopreserved then rapidly thawed.7 In the Figure, we show the standard surgical technique of laparoscopic orthotopic autotransplantation of cryopreserved-thawed ovarian tissue as performed in the Department of Obstetrics & Gynecology at the University of Cologne.8
FIGURE. Surgical Technique of Laparoscopic Orthotopic Autotransplantation of Cryopreserved-Thawed Ovarian Tissue
To date, autotransplantation of cryopreserved-thawed ovarian tissue has resulted in more than 120 live births worldwide.9 Due to its promising results, ovarian tissue cryopreservation and further autotransplantation was announced in 2019 as an established option for female fertility preservation by the American Society for Reproductive Medicine.10
However, the possible contamination of ovarian tissue with hematological malignant cells or minimal residual disease (MRD) remains a significant, serious concern regarding autotransplantation of cryopreserved-thawed ovarian tissue in patients with hematological malignancies. According to the results of several studies, systematic reviews, and meta-analyses designed to assess the risk of reintroducing hematological malignant cells, autotransplantation of cryopreserved-thawed ovarian tissue should be strongly contraindicated in all types of leukemia (high risk), although it may be performed in Hodgkin lymphoma (mild risk) and in non-Hodgkin lymphoma (moderate risk). Although that recommendation is reassuring for patients with lymphoma, autotransplantation of cryopreserved-thawed ovarian tissue should still be considered with caution for fertility restoration. To reduce the risk of reintroducing malignant cells, several tests should be performed in advance, including histological examination, in vitro culture, immunohistochemistry, polymerase chain reaction, multicolor flow cytometry, and long-term xenografting of ovarian tissue portion.11,12
When autotransplantation of cryopreserved-thawed ovarian tissue is contraindicated, that tissue could instead be processed in vitro for developing an artificial ovary; it would be an experimental option for fertility preservation and restoration. Artificial ovary is a novel experimental technology that aims to produce mature oocytes ready for in vitro fertilization through an ex vivo multistep strategy that includes sequential in vitro cultures of ovarian tissue, follicles, and oocytes.13-15 Artificial human ovary could be the safest option to preserve and restore fertility of young women and girls with hematological malignancies, especially when other fertility preservation options are not feasible or are contraindicated. Although artificial ovary has been successful in producing mature oocytes only in mice, to date, further research advances are likely to improve results and help to establish it successfully in humans.

Conflict of Interest: All authors state that they have no conflicts of interest.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
1. Jadoul P, Kim SS; ISFP Practice Committee. Fertility considerations in young women with hematological malignancies. J Assist Reprod Genet. 2012;29(6):479-487. doi:10.1007/s10815-012-9792-0
2. Salama M, Isachenko V, Isachenko E, Rahimi G, Mallmann P. Advances in fertility preservation of female patients with hematological malignancies. Expert Rev Hematol. 2017;10(11):951-960. doi:10.1080/17474086.2017.1371009
3. Salama M, Anazodo A, Woodruff TK. Preserving fertility in female patients with hematological malignancies: a multidisciplinary oncofertility approach. Ann Oncol. 2019;30(11):1760-1775. doi:10.1093/annonc/mdz284
4. Salama M, Mallmann P. Emergency fertility preservation for female patients with cancer: clinical perspectives. Anticancer Res. 2015;35(6):3117-3127.
5. Isachenko V, Todorov P, Isachenko E, et al. Cryopreservation and xenografting of human ovarian fragments: medulla decreases the phosphatidylserine translocation rate. Reprod Biol Endocrinol. 2016;10;14(1):79. doi:10.1186/s12958-016-0213-6
6. Van der Ven H, Liebenthron J, Beckmann M, et al; FertiPROTEKT Network. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031-2041. doi:10.1093/humrep/dew165
7. Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34(4):807-822. doi: 10.1007/s10555-015-9600-2
8. Isachenko V, Morgenstern B, Todorov P, et al. Patient with ovarian insufficiency: baby born after anticancer therapy and re-transplantation of cryopreserved ovarian tissue. J Ovarian Res. 2020;13(1):118. doi:10.1186/s13048-020-00713-9
9. Klüver Jensen A, Tryde Macklon K, Fedder J, Ernst E, Humaidan P, Yding Andersen C. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34(3):325-336. doi:10.1007/s10815-016-0843-9
10. Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112(6):1022-1033. doi:10.1016/j.fertnstert.2019.09.013
11. Bastings L, Beerendonk CCM, Westphal JR, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update. 2013;19(5):483-506. doi:10.1093/humupd/dmt020
12. Dolmans M-M, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99(6):1514-1522. doi:10.1016/j.fertnstert.2013.03.027
13. Amorim CA, Shikanov A. The artificial ovary: current status and future perspectives. Future Oncol. 2016;12(20):2323-2332. doi:10.2217/fon-2016-0202
14. Telfer EE, Fauser BCJM. Important steps towards materializing the dream of developing an artificial ovary. Reprod Biomed Online. 2016;33(3):333-334. doi:10.1016/j.rbmo.2016.07.005
15. Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. doi:10.1038/ncomms15261

Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.