Epirubicin May Offer Advantages Over Doxorubicin in Adjuvant Breast Cancer Treatment
TORONTO-Epirubicin (Ellence) and doxorubicin (Adriamycin) in combination regimens for adjuvant treatment of breast cancer will be compared in a major randomized controlled trial sponsored by the National Cancer Institute of Canada (NCIC), according to Maureen Trudeau, MD, head of systemic therapy at Sunnybrook Regional Cancer Centre in Toronto, Ontario. Dr. Trudeau described the reasoning behind and the design of NCIC CTG-MA.21 at a clinical investigators’ workshop sponsored by the University of Texas M. D. Anderson Cancer Center and Pharmacia Oncology.
TORONTOEpirubicin (Ellence) and doxorubicin (Adriamycin) in combination regimens for adjuvant treatment of breast cancer will be compared in a major randomized controlled trial sponsored by the National Cancer Institute of Canada (NCIC), according to Maureen Trudeau, MD, head of systemic therapy at Sunnybrook Regional Cancer Centre in Toronto, Ontario. Dr. Trudeau described the reasoning behind and the design of NCIC CTG-MA.21 at a clinical investigators workshop sponsored by the University of Texas M. D. Anderson Cancer Center and Pharmacia Oncology.
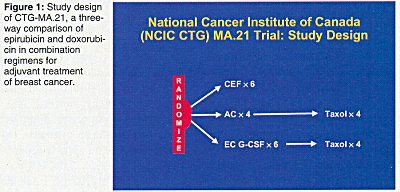
The Canadian study will compare cyclophosphamide (Cytoxan)/epirubicin/5-fluorouracil (CEF) to doxorubicin (Adriamycin)/cyclophosphamide (AC) plus paclitaxel (Taxol) and to epirubicin/cyclophosphamide (EC) plus paclitaxel in patients who have node-positive or high-risk node-negative breast cancer and have had total or partial mastectomies (see Figure 1).
National Variations
While AC plus paclitaxel is commonly used in the United States and CEF in Canada, many European centers favor EC as standard adjuvant therapy, according to Dr. Trudeau. One reason for this is the lower risk of cardiac toxicity associated with epirubicin. A number of studies have compared combinations including epirubicin and doxorubicin at equal doses. The FEC-50 regimen (5-FU, epirubicin 50 mg/m², cyclophosphamide) has been tested against the standard doxorubicin-containing FAC-50 regimen for metastatic breast cancer in several trials. According to Dr. Trudeau, response rates and median survival were comparable. The most important difference in these studies was the much lower cardiac toxicity associated with the epirubicin-containing regimens, she said.
In 1998, Ontario cancer specialists reviewed the clinical trials comparing epirubicin and doxorubicin in women with metastatic breast cancer. There were no significant differences in response rates or in median survival, and we concluded that these two agents are equally efficacious. Toxicity was significantly less with FEC than with FAC, Dr. Trudeau said. She also pointed out that it is possible to increase the epirubicin dose without being limited by the cardiac toxicity and hand-foot syndrome associated with high doses of doxorubicin.
Comparing Package Regimens

That overview inspired the development of the NCICs MA.5 trial, which directly compared CEF to CMF (cyclophosphamide, methotrexate, 5-fluorouracil) in patients with breast cancer. This trial is not comparing drug to drug, but package regimen to package regimen, Dr. Trudeau said (see Figure 2). The 710 patients were stratified by total vs partial mastectomy, hormone receptor status, and number of positive lymph nodes. Patients were randomized either to six 4-week cycles of CEF or CMF.
Dr. Trudeau reported that 5-year survival was significantly better with CEF than with CMF (77% vs 70%, P = .03). This represents a 19% reduction in the risk of death, she said.
CEF was associated with more neutropenia, fever or infection, thrombocytopenia, alopecia, and vomiting during the courses of treatment. The epirubicin-containing regimen was also associated with late adverse events Dr. Trudeau described as a bit disconcerting. These included congestive heart failure (1.1% vs 0.3% of patients), acute myelogenous leukemia (1.1% vs 0.3%), and acute lymphocytic leukemia (0.3% vs 0%).
Dr. Trudeau said that most patients in this trial were given trimethoprim-sulfamethoxazole as a prophylactic measure, and the researchers wonder if this antifolate might have contributed to the leukemia risk. Most patients now use ciprofloxacin (Cipro), and no further leukemias have been documented, she said.
Survival Higher with CEF
Relapse-free survival and overall survival were significantly improved in patients treated with CEF compared with CMF, Dr. Trudeau announced. CEF corresponded with a 29% risk reduction in recurrence and a 19% risk reduction in mortality, compared with CMF. Acute and delayed toxicities were manageable, and quality of life was clinically similar between the two treatments.
Consequently, the CEF regimen was used as the first arm in the new NCIC study. The second is the AC plus paclitaxel regimen reported in Cancer and Leukemia Group B (CALGB) 9344. The third is EC followed by paclitaxel. About 1,500 patients will be randomized among these three arms.
Previous studies showed that CEF is superior to CMF, that AC followed by paclitaxel is superior to AC, and that CEF is probably equivalent to AC followed by paclitaxel, Dr. Trudeau said. Adding a taxane after 6 months of CEF was too difficult. Studies also suggest that 3 months of EC with G-CSF support is equivalent to 6 months of CEF. The new EC treatment uses dose-dense, dose-intense chemotherapy with a sequenced taxane and will be compared to the standard US and Canadian aggressive treatments, CEF and AC followed by paclitaxel, Dr. Trudeau concluded.