Irinotecan/Gemcitabine Combination Advances to Phase III Trials in Advanced Pancreatic Cancer
CHARLESTON, South Carolina-‘‘Highly censored data” from a multicenter phase II trial of irinotecan (Camptosar)/gemcitabine (Gemzar) suggest that this combination, known as IrinoGem, “is well tolerated and active in advanced and metastatic pancreatic cancer,” Caio Max S. Rocha Lima, MD, reported at a clinical investigators’ workshop. IrinoGem is now being compared to gemcitabine alone in an international multicenter phase III randomized trial involving 75 institutions and 350 patients with locally advanced or metastatic pancreatic adenocarcinoma.
CHARLESTON, South CarolinaHighly censored data from a multicenter phase II trial of irinotecan (Camptosar)/gemcitabine (Gemzar) suggest that this combination, known as IrinoGem, is well tolerated and active in advanced and metastatic pancreatic cancer, Caio Max S. Rocha Lima, MD, reported at a clinical investigators workshop. IrinoGem is now being compared to gemcitabine alone in an international multicenter phase III randomized trial involving 75 institutions and 350 patients with locally advanced or metastatic pancreatic adenocarcinoma.
Irinotecan and gemcitabine are also potent radiation sensitizers, and clinical trials exploring IrinoGem plus radiation therapy in patients with advanced pancreatic cancer are also warranted, Dr. Rocha Lima told participants of the workshop, sponsored by the University of Texas
M. D. Anderson Cancer Center and Pharmacia Oncology. Dr. Rocha Lima is Assistant Professor of Medicine at the Medical University of South Carolina in Charleston, South Carolina.
Single-Agent Activity
Each drug is active as a single agent in a variety of solid tumors and produces similar response rates and median survivals in advanced and metastatic pancreatic cancer. The agents have complementary rather than overlapping toxicity and showed synergy in preclinical studies, Dr. Rocha Lima reported.
In the phase I study of irinotecan/gemcitabine in solid tumors, maximum tolerated doses were 100 mg/m² of irinotecan and 1,000 mg/m² of gemcitabine. Both drugs were given on days 1 and 8 every 3 weeks. This permitted a potential for 100% of single-agent irinotecan dose intensity and 80% of single-agent gemcitabine dose intensity and avoided day 15 treatment. That day is associated with frequent dose omissions/reductions on previously tested weekly gemcitabine combinations. There were three partial responses in the phase I trial.
Phase II Results
Based on the promising phase I data, Dr. Rocha Lima and colleagues conducted a phase II study in 45 patients with previously untreated advanced and metastatic pancreatic cancer. The two drugs were again given on days 1 and 8, with the cycle repeating on day 21.
Treatments administered totaled 394, with 89% dose intensity for irinotecan and 87% for gemcitabine. There was modest toxicity with no toxic deaths, Dr. Rocha Lima said. There was no grade 4 diarrhea, but grade 3 diarrhea occurred in 6.7% of patients. Grade 4 neutropenia occurred in 2.2% of patients. There was no febrile neutropenia, but grade 3 neutropenia occurred in 15.6% of patients.
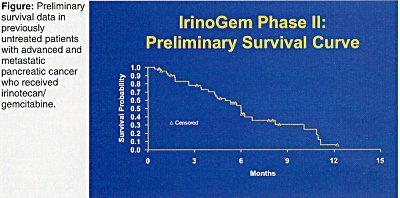
Overall response rate was 20%. CA19 levels dropped by more than half in 38% of patients. Time to treatment failure was 2.8 months, and median survival was 6.0 months (range 0.3 to 15.5 months) (see Figure). This combination is well tolerated and active in metastatic pancreatic cancer. The ongoing phase III trial will help define the role of IrinoGem in pancreas cancer, Dr. Rocha Lima concluded.