Erlotinib/Gemcitabine for First-Line Treatment of Locally Advanced or Metastatic Adenocarcinoma of the Pancreas
Erlotinib (Tarceva) is a human epidermal growth factor receptor type 1/epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor initially approved by the US Food and Drug Administration for the treatment of patients with locally advanced or metastatic non–small-cell lung cancer after failure of at least one prior chemotherapy regimen. In this report, we present the pivotal study that led to the approval of erlotinib in combination with gemcitabine (Gemzar) in patients with locally advanced/metastatic chemonaive pancreatic cancer patients. The combination demonstrated a statistically significant increase in overall survival accompanied by an increase in toxicity. Physicians and patients now have a new option for the treatment of locally advanced/metastatic adenocarcinoma of the pancreas.
Erlotinib (Tarceva) is a human epidermal growth factor receptor type 1/epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor initially approved by the US Food and Drug Administration for the treatment of patients with locally advanced or metastatic non–small-cell lung cancer after failure of at least one prior chemotherapy regimen. In this report, we present the pivotal study that led to the approval of erlotinib in combination with gemcitabine (Gemzar) in patients with locally advanced/metastatic chemonaive pancreatic cancer patients. The combination demonstrated a statistically significant increase in overall survival accompanied by an increase in toxicity. Physicians and patients now have a new option for the treatment of locally advanced/metastatic adenocarcinoma of the pancreas.
The prognosis of advanced pancreatic carcinoma has not improved in the past 20 years. The most recent drug approved by the US Food and Drug Administration (FDA) for the treatment of advanced pancreatic carcinoma was gemcitabine (Gemzar), approximately 10 years ago. The approval was based on two clinical trials in patients with locally advanced or metastatic pancreatic cancer. In the multicenter, prospective, single-blinded, two-arm, randomized pivotal trial, chemonaive patients received gemcitabine or fluorouracil (5-FU).[1,2] Patients treated with gemcitabine not only had a statistically significant increase in clinical benefit response (a composite endpoint that reflects a measure of clinical improvement based on analgesic consumption, pain intensity, performance status, and weight change) but also experienced increases in survival and time to disease progression compared to 5-FU. This increase in survival was not associated with any evidence of tumor response.[2]
Although initial efforts to combine gemcitabine with other agents failed to demonstrate significant benefit, a few recent studies suggested that some gemcitabine-containing combination regimens may benefit patients with advanced adenocarcinoma of the pancreas.[3,4] Moreover, a recent meta-analysis[5] suggests that gemcitabine combinations improve overall survival compared with gemcitabine alone. Despite all these efforts, novel therapies are desperately needed for the treatment of this disease.
Erlotinib (Tarceva; OSI Pharmaceuticals, Inc, Melville, NY, and Genentech, Inc, South San Francisco, Calif) inhibits epidermal growth factor receptor (EGFR) tyrosine kinase (TK) autophosphorylation by inhibition of the intracellular domain. Studies in cell lines and enzyme assays have both shown that erlotinib inhibits EGFR at concentrations significantly lower than those needed to inhibit c-src and v-abl.[6] While erlotinib was more selective for EGFR TK than it was for several other tyrosine kinases tested, several other TKs in the same family as EGFR may be sensitive to erlotinib. On November 2004, erlotinib received FDA approval as monotherapy for the treatment of patients with locally advanced or metastatic non–small-cell lung cancer (NSCLC) after failure of at least one prior chemotherapy regimen.[7,8]
Survival of erlotinib-treated patients was superior to that of placebo-treated patients. Exploratory univariate analyses showed a larger survival prolongation in two subsets of patients: those who never smoked and those with EGFR-positive tumors. Moreover, patients who developed rash during therapy demonstrated better survival.[7,8] Skin rash and diarrhea were the most common erlotinib adverse events (AEs). In order to increase the antitumor effect of erlotinib, several combinations were tested in patients with lung cancer. In two large controlled, randomized trials in first-line NSCLC patients, the addition of erlotinib to doublet, platinum-based chemotherapy did not show any benefit.[9,10] Therefore, erlotinib is not indicated for use in this setting.
In this report, we present the pivotal study that led to the approval of erlotinib in combination with gemcitabine in patients with locally advanced/metastatic chemonaive pancreatic cancer patients. The combination demonstrated a statistically significant increase in overall survival accompanied by an increase in toxicity. This is the first time that a small-molecule TK inhibitor demonstrated an increase in survival when combined with chemotherapy for the treatment of solid tumors. The combination of gemcitabine and erlotinib is a new treatment option for this patient population.
Patients and Methods
Patients
Eligible patients had histologically or cytologically confirmed adenocarcinoma of the pancreas that was unresectable, locally advanced, or metastatic. No prior systemic chemotherapy was allowed. However, patients could have received prior radiation treatment for management of local disease providing that disease progression had been documented, all toxicities had resolved, and the last fraction of radiation treatment was completed at least 4 weeks prior to randomization. Patients were not eligible if they had received prior chemotherapy other than 5-FU (with or without folinic acid) or gemcitabine given concurrently with radiation treatment as a radiosensitizer. Patients were required to have adequate renal, liver, and bone marrow reserve and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2.
Design
This was a randomized, double-blind, placebo-controlled, multi-institutional phase III study of erlotinib plus gemcitabine (EG) vs gemcitabine plus placebo (PG) in patients with incurable advanced or metastatic pancreatic cancer. This multinational trial was conducted by the National Cancer Institute of Canada Clinical Trials Group in collaboration with OSI Pharmaceuticals, Inc.[11]
Although the dose of erlotinib used at selected Canadian centers was 150 mg po qd, the dose used worldwide was 100 mg po qd. Because of the small number of patients in the 150-mg groups (48 on the EG arm and 24 on the PG arm), this review will discuss the results for the 100-mg group only.
A total of 521 patients were randomized to gemcitabine plus erlotinib (n = 261, EG arm) or to gemcitabine plus placebo (n = 260, PG arm) in a 1:1 ratio. Randomization was stratified by center, performance status (ECOG 0/1 vs 2), and extent of the disease (locally advanced vs distant metastases). Efficacy was evaluated by periodic assessments of survival. In addition, serial measurements of all disease sites were performed every 8 weeks, and tumor response was assessed using the response evaluation criteria in solid tumors (RECIST).
Safety was assessed every 4 weeks by evaluating changes in hematology and biochemistry parameters, changes in physical examination, and by monitoring the incidence, severity, and relationship of adverse events. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria (NCI CTC), version 2.0. After discontinuing protocol treatment, patients were evaluated at 4 weeks, and survival status was assessed every 12 weeks until death.
Efficacy Endpoints
The primary endpoint was overall survival. Secondary endpoints were tumor response, tumor response duration, and progression-free survival. The expression of EGFR levels at diagnosis was correlated with outcomes and response to treatment.
Statistical Methods
The primary survival analysis was survival time, defined as time from randomization to death, using the log-rank test stratified by the randomization stratification factors (extent of the disease [locally advanced vs distant metastases] and ECOG performance status [0/1 and 2]). Median survival was estimated using Kaplan-Meier estimates, and the 95% confidence interval was computed using the method of Brookmeyer and Crowley. No interim efficacy analysis was conducted in this study.
Analysis of a secondary endpoint, progression-free survival (defined as the time from randomization to the first observation of disease progression or death due to any cause), was conducted using the stratified log-rank test. Response rate was defined as the proportion of patients evaluable for response who met the criteria of complete or partial response. The Fisher's exact test (2-sided) was used to compare tumor response rates between the two treatment arms.
Results
Patient Baseline Characteristics
TABLE 1
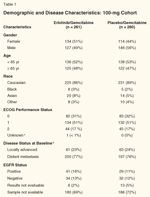
Demographic and Disease Characteristics: 100-mg cohort
Baseline demographic and disease characteristics of the 100-mg cohort are shown in Table 1. The initial sample size estimate was based on 381 events for the final analysis. However, a total of 569 patients were randomized and 551 events had occurred at the time of final analysis, including the 100- and 150-mg erlotinib cohorts.
In the 100-mg cohort, a total of 261 patients were randomized to the EG group and 260 patients were randomized to the PG group. Treatment groups were well balanced with respect to baseline characteristics (see Table 1). Only 17% of patients had ECOG PS 2. Also, half of the patients had a pain intensity score ≤ 20 (data not shown). Of note, PS 2 and pain intensity scores ≥ 20 portend poor prognosis. Median age was 64 and 63 years old, respectively, in the EG and PG treatment groups. With respect to extent of disease at baseline, approximately 25% had locally advanced pancreatic carcinoma. Most patients were < 6 months from time of initial diagnosis to randomization. Almost all patients had surgery before therapy. This includes any surgical procedure such as biopsy of the pancreas.
Approximately one-third of the patients were diagnosed by cytology (fine-needle aspiration), as can be expected for this type of tumor. Approximately 8% of patients received radiation therapy alone or in combination with radiation sensitization doses of chemotherapy.
The expression of EGFR (as measured by immunohistochemistry [IHC]), the main target for erlotinib, is presented in Table 1. A positive EGFR expression was defined as having at least 10% of tumor cells staining for EGFR using the DAKO EGFR pharmDX kit. Pathology blocks or slides were available and the results were interpretable for only 27% of patients in the EG arm and for 24% of patients in the PG arm. In the EG arm, 16% of patients' tumors (representing 56% of patients with known EGFR status) had a positive EGFR expression and 13% (44% of patients with known status) had a negative expression, compared with 11% and 12% (representing 46% and 54% of patients with known status) in the placebo arm. Of note, tissue collection was not a mandatory inclusion criterion.
TABLE 2

Causes of Ineligibility Due to Pathology Evaluation
In a blinded fashion, the FDA assessed the eligibility criteria for all 521 cases. Two FDA reviewers assessed the available pathology, surgical, and radiologic reports for all patients. In 18 cases, the diagnosis of pancreatic carcinoma was incorrect or unproven. Based on this information, the FDA performed sensitivity analyses excluding these ineligible patients (Table 2). The major protocol violations that the FDA observed were as follows: no pathology reports (n = 2), lack of confirmation of malignancy (n = 3), metastatic disease without proof of pancreatic origin as determined by computed tomography or surgical reports (n = 3); and other primary malignancy in the biopsy report (n = 10).
Cases with other primary malignancies were ampula of vater (n = 5), acinar cell carcinoma (n = 2), and adenocarcinoma of nonpancreatic origin, colon cancer, or gastric cancer (1 case each). Thus, there were three analysis populations for this study (see Table 3): all randomized patients (n = 521); analysis population excluding major protocol violations (n = 503), and safety population (patients who received study drug, n = 515). Patient disposition is described in Table 4. Of note, 62% discontinued due to disease progression in the EG arm as compared to 71% in the PG arm. However, a higher number of patients in the EG arm had discontinuation of drug due to adverse events, patient refusal or death.
Efficacy Analysis
TABLE 3

Populations for AnalysisTABLE 4

Patient DispositionTABLE 5

Sensitivity Analysis (Overall Survival Analysis Population Minus 18 Patients Without Pathology Confirmation)TABLE 6

Secondary Endpoints
• Survival by Treatment-When the application was submitted to the FDA in June 2005, the applicant submitted results from a survival analysis with 484 deaths with a data cutoff of September 17, 2004. At this time an excess of more than 100 deaths over the original plan for this analysis (n = 381) had occurred. The applicant provided an update with a data cutoff of June 2005. At that time, 504 patients had died and 17 patients were alive.
FIGURE 1
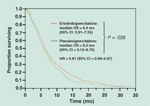
Overall Survival
An analysis performed by the FDA after 504 deaths revealed that the median overall survival was 6.4 months in the EG arm and 6.0 months in the PG arm (P = .03; see Figure 1 and Table 5). When overall survival was analyzed at 381 events, as planned in the original protocol, similar results were obtained: median survivals were 6.5 months (95% confidence interval [CI] = 5.95–7.36) and 6.0 months (95% CI = 5.09–6.70) with a P value of .06 (Table 5).
• Survival in Subgroups: Exploratory Univariate Analyses-The positive primary univariate analysis provided an opportunity to examine the prognostic effects of the stratification factors that were included in this analysis. In univariate analyses, both ECOG PS 2 and disease with distant metastases were associated with worse survival irrespective of treatment received. In the multivariate analysis, after adjustment for the treatment effect, both factors remained statistically significant (P < .001).
FIGURE 2

Survival Hazard Ratio in Subgroups Based on Pretreatment Characteristics
Most subsets analyzed showed some trend for benefit in the EG arm (see Figure 2). However, in patients with pain intensity > 20, female gender, age ≥ 65 years, or PS 0 or 1, the benefit of erlotinib was unclear (Figure 2). Interestingly, any potential survival benefit from EG does not seem to be related to EGFR expression status (see below).
• Sensitivity Analysis Excluding the 18 Ineligible Patients-When 18 patients without documentation of adenocarcinoma of the pancreas (as agreed by the applicant and FDA) were excluded, the nominal P values ranged from 0.06 in the 381-death group to 0.04 in the 443-death group.The hazard ratios were similar in all groups (Table 5).
FIGURE 3

Survival Analysis Based on EGFR Status
• Survival by Expression of EGFR in Tumor Samples-As demonstrated in Figure 3 (panel A and B) and Table 6, the expression of EGFR by IHC does not predict an increase in overall survival. However, caution must be applied because of the small number of patients in the groups with available EGFR status.
FIGURE 4

Survival Analysis by Incidence of Rash
• Exploratory Analysis of the Relationship Between Rash and Survival-In the pivotal NSCLC study, patients who developed rash had longer overall survival compared to patients without rash. Moreover, the higher the grade of rash, the larger the improvement in overall survival.[7,8] An exploratory analysis was performed in order to determine whether rash predicted the outcome in this trial. Although patients with ≤ grade 1 rash (Figure 4, panel B) did not have any prolongation in survival with erlotinib, patients with ≥ grade 2 (Figure 4, panel A) had a significant increase in OS when treated with EG. Thus, patients with ≥ grade 2 rash (eg, maculopapular symptomatic rash), may benefit from EG. These results must be interpreted with caution but appear to suggest that patients with minimal or no rash (< grade 2) may not benefit from EG therapy.
• Progression-Free Survival-PFS was a secondary endpoint defined as the time from randomization to the first observation of disease progression or death due to any cause. For the PFS analysis, ineligible cases due to lack of pathologic confirmation were not excluded. A statistically significant difference was seen between the two treatment arms with respect to the secondary endpoint, PFS (Table 6). Median PFS was 3.8 months (95% CI = 3.6–4.9) in the EG group vs 3.5 months (95% CI = 3.3–3.7) in the PG group, with a hazard ratio of 0.76 (95% CI = 0.64–0.92, adjusted log-rank P value = .006). A total of 64 patients were censored at the time of analysis (EG arm, n = 36 and PG arm, n = 28).
• Objective Tumor Responses and Duration of Responses-A total of 1 complete response (CR) and 20 partial responses (PR), determined by RECIST criteria, were observed in the EG arm and a similar number-2 CRs and 17 PRs-were observed in the PG arm, for an overall objective response rate of 8.6% (95% CI = 5.4–12.9) in the EG arm and 7.9% (95% CI = 4.8–12.0) in the PG arm, P = .869 (Table 6). Thus, no differences were observed in objective response rates between the EG and PG arms. Moreover, there were no differences in duration of response. The median duration of the objective responses (CR and PR) was 23.9 weeks (95% CI = 16.3–29.6) in the EG arm and 23.3 weeks (95% CI = 16.1–32.4) in the PG arm (Table 6).
Safety Analyses
TABLE 7

Overall Safety
A total of 521 patients were randomized to 100 mg erlotinib (n = 261) or to placebo (n = 260). Of these patients, a total of 515 patients received at least one dose of either erlotinib (n = 259) or placebo (n = 256). As shown in Table 7, a higher proportion of patients receiving EG (n = 81, 31%) died on treatment or within 30 days of last treatment compared to placebo (n = 68, 27%). A higher proportion of patients in the EG group had adverse events leading to discontinuation (Table 7). Moreover, serious adverse events and ≥ grade 3 treatment-related adverse events were more frequent on the EG arm than the PG arm.
TABLE 8

Causes of Death in Patients Who Died Within 30 Days of Last Dose of Treatment
Death within 30 days of drug administration was attributed to toxicity or pancreatic cancer plus protocol treatment in 5 patients (Table 8), all in the erlotinib arm (6.2% vs 0%). The events resulting in death included 2 cases of pneumonitis, neutropenic and nonneutropenic sepsis in 1 patient each, and 1 case of CNS bleeding. Moreover, 3 additional patients experienced serious drug-related events with a fatal outcome. In contrast, a lower percentage of deaths due to pancreatic cancer progression was observed in the EG arm.
TABLE 9

Severe Adverse Events (NCI CTC ≥ Grade 3) Occurring in 10% or More of Erlotinib-Treated Patients, Regardless of CausalityTABLE 10
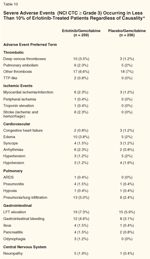
Severe Adverse Events (NCI CTC ≥ Grade 3) Occurring in Less Than 10% of Erlotinib-Treated Patients, Regardless of Causality
• Severe Adverse Events-Severe adverse events (NCI CTC ≥ grade 3 severity) are shown in Tables 9 and 10. Table 9 lists the AEs that were observed in ≥ 10% of erlotinib-treated patients. Table 10 lists the AEs observed in < 10% of erlotinib-treated patients. The EG group had a slightly higher incidence of severe ≥ grade 3 AEs including rash, diarrhea, infections, and bone pain (Table 9). In Table 10, other ≥ grade 3 AEs that were more frequent in the EG group included stroke, cardiac ischemia/infarction, acute respiratory distress syndrome, pneumonitis, deep venous thrombosis (DVT), edema, arrhythmias, ileus, pancreatitis, odynophagia/stomatitis, thrombocytopenia, neuropathy, and renal failure.
Six patients in the EG group developed stroke (2.3%). One of these strokes was hemorrhagic and fatal. In comparison, there were no strokes in the PG group. The median time to stroke was 24 days (95% CI = 12–36). The earliest case of stroke occurred by 2 days after drug initiation, and the latest was 35 days after drug initiation.
In the erlotinib group, 6 patients developed myocardial ischemia/infarction (incidence of 2.3%). One of these patients died due to a myocardial infarction (MI). In comparison, 3 patients the PG group developed myocardial ischemia/infarction (1.2%) and 1 died due to MI. The median time to onset of MI in the EG group was 72 days (95% CI = 21–123). The earliest case occurred 13 days after drug initiation, and the latest was 212 days after drug initiation.
Patients in the EG group had 6 episodes of ≥ grade 3 arrhythmia, including 2 sinus tachycardia, 2 atrial fibrillation, 2 undefined tachycardia and arrhythmia episodes, for an incidence of 2.3%. In contrast, the incidence in the PG group was 0.8 %. One case of atrial fibrillation and one case of sinus tachycardia occurred in the PG group.
Although a similar number of patients had significant congestive heart failure, the EG arm had a higher incidence of ≥ grade 3 edema (3.8 % vs 2 %, respectively).
Ten patients in the EG group developed DVT (3.9%). In comparison, only 3 patients in the PG group developed DVT (1.2%). The overall incidence of grade 3 or 4 thrombotic events, including DVT, was slightly higher in the EG arm: 11% for erlotinib plus gemcitabine and 9% for placebo plus gemcitabine.
Two patients developed microangiopathic hemolytic anemia with thrombocytopenia (thrombotic thrombocytopenic purpura [TTP]-like disease) in the EG group (0.8%) compared to no patients in the PG group. Three additional cases of TTP-like disease have been reported during postmarketing experience.
Although the number of sepsis episodes was similar (22 cases in each group), the number of "other severe infections" was higher in the EG group (13 vs 2). The list of other severe infections in EG recipients included 5 cholangitis, 3 cellulitis, 2 wound infections, 1 peritonitis, 1 salmonellosis, 1 urosepsis, and 1 vaginal infection. In contrast, the PG group had 1 episode of cholangitis and 1 wound infection. Three patients in the EG group (1 neutropenic sepsis, 1 undefined sepsis, and 1 cholangitis) and two patients in the PG group died due to sepsis.
Ten patients in the EG group developed ≥ grade 3 pneumonia (4.0%) whereas only five patients developed pneumonia in the PG group (2 %). Two patients with pneumonia died in the EG group, while no patient died due to pneumonia in the PG group. Another significant adverse event is interstitial lung disease (ILD)-like pulmonary toxicity. This adverse event was previously reported in the pivotal lung cancer study.[7,8] A total of 7 patients developed serious ILD-like events-6 in the EG arm (2.3%) and 1 in the PG arm (0.4%). Three patients in the EG group but none in the PG group died due to ILD-like events. The median time to an ILD-like event was 50 days (95% CI = 17–63). The earliest case of an ILD-like event occurred by 39 days after drug initiation, and the latest case came 122 days after drug initiation. The incidence of 2.3% in the EG arm is higher than that observed with erlotinib monotherapy in the NSCLC pivotal trial (0.8%).
A well recognized adverse event associated with erlotinib is rash. In this study, 12 cases of severe rash were observed in the EG group (4.6 %). In contrast, only 3 patients had severe rash in the PG group (1.2%). The median time to onset for all grades of rash was 10 days. However, median time to onset for ≥ grade 2 rash was 8 days.
Severe GI bleeding was observed in 12 (4.6 %) and 8 (3.1%) of patients in the EG and PG arm, respectively. Two cases were fatal, all in the EG group. No patient died due to GI bleeding in the placebo group.
Discussion
In this trial, the combination of erlotinib and gemcitabine demonstrated a modest increase in overall survival compared to gemcitabine alone. The increase in survival was accompanied by an increase in PFS. However, the response rate and response duration were not different between groups. Moreover, patients receiving the combination of EG demonstrated an increase in toxicity as manifested by increased discontinuation due to drug toxicity, an increased number of toxic deaths, and increased serious adverse events and severe toxicities.
When the study was conducted, gemcitabine had been the most recently approved agent for the treatment for advanced pancreatic carcinoma. In the pivotal trial that led to the gemcitabine approval, an increase in overall survival and improvement in clinical benefit response was established.[2] Over the past few years, several gemcitabine-containing regimens were tested in this setting. Although initially most trials of gemcitabine combinations failed to show a survival advantage over gemcitabine alone, several recent publications have reported a benefit for such combinations.[3,4]
Xie et al[5] performed a meta-analysis using all available randomized clinical trials (19 trials, 1996–2004) with gemcitabine-based combination chemotherapy vs gemcitabine alone in advanced or metastatic pancreatic cancer. According to this report, the gemcitabine-based combinations produced a significant improvement in overall survival at 6 months and 1 year. The investigators also reported a statistically significant increase in response rate, PFS, and TTP at 6 months. Moreover, they found a trend in favor of the combination regimens for clinical response improvement. Thus, this meta-analysis, along with recent reports, suggests that combination gemcitabine chemotherapy may prolong overall survival.
Several trials using small-molecule EGFR TK inhibitors combined with chemotherapy were reported with negative results. For example, two large placebo-controlled studies in over 2,000 patients with advanced NSCLC without prior chemotherapy comparing carboplatin and paclitaxel with or without concurrent erlotinib and gemcitabine, and cisplatin with or without concurrent erlotinib, showed no improvement in survival, PFS, or tumor response with the use of erlotinib.[9,12] Other studies using another EGFR TK inhibitor, gefitinib (Iressa), demonstrated similar negative results when used in combination with chemotherapy. Thus, it appears that this is the first trial to show an additive effect of chemotherapy and a small-molecule EGFR TK inhibitor.
Although erlotinib was developed to target the EGF receptor, the expression of EGFR in the monotherapy NSCLC trial did not predict any benefit.[8,10] A predictor of survival in the NSCLC monotherapy study was the incidence of rash. Patients who developed rash of any grade appeared to have an increase in overall survival. In contrast, in the pancreatic study, only patients with severe (≥ grade 2) rash appeared to have an increase in survival. However, these analyses are exploratory and should be interpreted with caution.
Although erlotinib monotherapy (150 mg po qd) appears to be well tolerated, the combination of 100 mg of erlotinib and standard doses of gemcitabine appears to be significantly more toxic than gemcitabine alone.[11] Notwithstanding the limitation of cross-study comparisons, the combination of EG also appears to be significantly more toxic than erlotinib monotherapy.[13] Several recognized toxicities such as ILD-like events (2.3%) or liver function test elevation (7.3%) were greater with the combination, compared to erlotinib monotherapy (in the NSCLC monotherapy study). Moreover, new and previously unrecognized toxicities-such as stroke, peripheral neuropathy, arrhythmias, MI, ileus, edema, pancreatitis, renal failure, and microangiopathic hemolytic anemia with thrombocytopenia-were greater with the EG combination.
Because of the modest survival improvement and increase in toxicity observed in this trial, this application was discussed at the Oncologic Drugs Advisory Committee (ODAC) meeting in September 2005. ODAC voted in favor of approval (10 yes and 3 no). FDA followed the advice of ODAC and approved the application in November 2005.
In summary, the addition of erlotinib to gemcitabine is associated with increases in overall survival and PFS in patients with locally advanced, unresectable, or metastatic pancreatic cancer. Although these increases are modest and the combination is more toxic, this is the first trial since the gemcitabine approval to show an increase in survival in this population. Moreover, this is the first clinical trial of a small-molecule EGFR TK inhibitor that increases survival in solid tumor patients when combined with chemotherapy. Thus, therapy with this combination should be considered in patients with locally advanced, unresectable, or metastatic pancreatic cancer.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Acknowledgements:The authors would like to acknowledge Drs. Lourdes Villalba and Ramzi Dagher for their review and suggestions in this manuscript.
References:
1. Rothenberg ML, Moore MJ, Cripps MC, et al: A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol 7:347-353, 1996.
2. Burris HA 3rd, Moore MJ, Andersen J, et al: Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 15:2403-2413, 1997.
3. Reni M, Cordio S, Milandri C, et al: Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: A randomised controlled multicentre phase III trial. Lancet Oncol 6:369-376, 2005.
4. Louvet C, Labianca R, Hammel P, et al: Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509-3516, 2005.
5. Xie DR, Liang HL, Wang Y, et al: Meta-analysis on inoperable pancreatic cancer: A comparison between gemcitabine-based combination therapy and gemcitabine alone. World J Gastroenterol 12:6973-6981, 2006.
6. Herbst RS: Erlotinib. Clin Adv Hematol Oncol 3:125, 141, 2005.
7. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al: Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123-132, 2005.
8. Johnson JR, Cohen M, Sridhara R, et al: Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 11:6414-6421, 2005.
9. Perez-Soler R: The role of erlotinib (Tarceva, OSI 774) in the treatment of non-small cell lung cancer. Clin Cancer Res 10:4238s-4240s, 2004.
10. Shepherd FA: Second-line chemotherapy for non-small cell lung cancer. Expert Rev Anticancer Ther 3:435-442, 2003.
11. Moore MJ, Goldstein D, Hamm J, et al, for the National Cancer Institute of Canada Clinical Trials Group: Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960-1966, 2007.
12. Herbst RS, Prager D, Hermann R, et al: TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23:5892-5899, 2005.