Erythropoiesis-Stimulating Protein Support and Survival
Anemia is common in many patients with cancer treated with chemotherapy. One option for managing chemotherapy-induced anemia (CIA) is erythropoiesis-stimulating proteins (ESPs), which are indicated for the treatment of CIA in patients with most types of cancer. They have been shown to be safe and effective in numerous well-documented studies, and their side effects are well known. The rate of thrombotic events with the long-acting ESP darbepoetin alfa (Aranesp) has been consistent in studies conducted before and after its approval. The association of thrombotic events with high hemoglobin levels or rapid increases in its levels in patients with cancer remains controversial. Adjusting the dose of the ESP to maintain and monitor a target hemoglobin level of 11 to 12 g/dL is certainly prudent and may help prevent or minimize these events. Chemotherapy-induced anemia has been associated with shorter survival in patients with cancer, and the relation is likely multifactorial. Data on the treatment of CIA with ESPs have not shown a consistent effect on survival. Two studies in patients with hemoglobin levels above the target level showed that survival was shorter in the patients treated with ESPs. A review of data from other trials found no effect of ESPs on survival, and other trials suggested a positive effect. This article reviews data on survival in patients treated with ESPs and discusses five large randomized controlled trials of darbepoetin alfa that are addressing this issue.
ABSTRACT: Anemia is common in many patients with cancer treated with chemotherapy. One option for managing chemotherapy-induced anemia (CIA) is erythropoiesis-stimulating proteins (ESPs), which are indicated for the treatment of CIA in patients with most types of cancer. They have been shown to be safe and effective in numerous well-documented studies, and their side effects are well known. The rate of thrombotic events with the long-acting ESP darbepoetin alfa (Aranesp) has been consistent in studies conducted before and after its approval. The association of thrombotic events with high hemoglobin levels or rapid increases in its levels in patients with cancer remains controversial. Adjusting the dose of the ESP to maintain and monitor a target hemoglobin level of 11 to 12 g/dL is certainly prudent and may help prevent or minimize these events. Chemotherapy-induced anemia has been associated with shorter survival in patients with cancer, and the relation is likely multifactorial. Data on the treatment of CIA with ESPs have not shown a consistent effect on survival. Two studies in patients with hemoglobin levels above the target level showed that survival was shorter in the patients treated with ESPs. A review of data from other trials found no effect of ESPs on survival, and other trials suggested a positive effect. This article reviews data on survival in patients treated with ESPs and discusses five large randomized controlled trials of darbepoetin alfa that are addressing this issue.
Anemia is common in patients with cancer who are treated with chemotherapy.[1] In addition to the physical symptoms and the diminished quality of life in patients with anemia, there is also evidence that the greater tumor hypoxia due to anemia may result in a poorer response to chemotherapy. Anemia is a risk factor for early mortality in nearly all cancers in which it has been studied, with a hazard ratio (HR) of 1.65.[2] Several preclinical studies have found correlations between anemia, tumor oxygenation, tumor response, and survival.[3-5] Strategies to lessen chemotherapy-induced anemia (CIA) may not only alleviate anemia-related symptoms and improve quality of life, but also increase tumor responses and possibly increase survival. Erythropoiesis-stimulating proteins (ESPs) are indicated for the treatment of CIA in patients with most types of cancer.
The efficacy and safety of ESPs have been well studied, but there are questions about their association with thrombotic events and survival. An association of higher thrombotic rates with high hemoglobin (Hgb) levels or a rapid rise in Hgb level has been described in patients who undergo renal dialysis. This is implicated in the population of patients with cancer, in whom the thrombotic risk is increased by the cancer, its treatment, and comorbidities. In trials in patients with CIA treated with ESPs, the risk of thrombotic events was identified. These rates have remained consistent across preapproval and postapproval studies.
There has also been concern that treatment with ESPs may promote tumor growth, possibly owing to the greater delivery of oxygen to tumor cells as a result of higher Hgb levels.[6] The binding of ESPs to erythropoietin receptors may also promote the proliferation of endothelial cells, tumor angiogenesis, and vasculogenesis. Preclinical studies have reported high levels of erythropoietin and erythropoietin receptors in tumor cells. Erythropoiesis-stimulating proteins may directly promote tumor proliferation and survival of tumor cells that express erythropoietin receptors. Two clinical studies have shown lower survival in patients treated with ESPs,[7,8] but other data indicate that treatment with ESPs has either no effect or the possibility of a beneficial effect on survival.[9] To address the issue of ESP tumor-related stimulation, most studies have not shown a detrimental effect on tumor progression with treatment for anemia. In this article, I review data on survival in patients treated with ESPs and discuss five ongoing large randomized controlled trials that are investigating the association between treatment with ESPs and survival.
Lower Survival in Patients Treated with ESPs
Two studies have reported lower survival in patients with anemia treated with ESPs than in those treated with placebo[7,8]; both trials were terminated early for that reason. One of these trials[7] investigated the effect of the use of ESPs to maintain normal Hgb levels (> 12 g/dL and < 14 g/dL) on survival in 939 patients with metastatic breast cancer and Hgb levels of 13 g/dL. This trial was to have lasted 12 months, but it was terminated early because of early mortality, particularly in the first 4 months. Survival at 12 months was 6 percentage points higher with placebo than with epoetin alfa (Epogen) (76% vs 70%; P = .012). However, follow-up beyond the treatment period, at 19 months, showed convergence of the survival rates.
In an analysis of early death rates, there was a higher incidence of disease progression in the epoetin alfa group than in the placebo group (6% vs 3%) and a greater incidence of thrombotic and vascular events (1% vs 0.2%). However, time to disease progression was not different in the two groups. In addition, the results in this trial may not be conclusive because of imbalances between the epoetin alfa and placebo groups. Only the presence of metastases was stratified, and not at every study center. Characteristics such as prognostic factors, risk factors, age, performance status, extent of disease at study entry, and risk factors for thrombovascular events were not balanced in the two groups.
Another randomized, double-blind, placebo-controlled trial also found lower survival with ESPs than with placebo.[8] This study investigated whether treatment with epoetin beta could increase the control of cancer and survival in 351 patients with head and neck cancer. Treatment with epoetin beta did increase Hgb levels, but tumor control and survival were not greater. The Hgb levels were ≥ 14.5 g/dL in 82% of the patients treated with epoetin beta but in only 15% of those treated with placebo. Overall survival was significantly greater in patients treated with placebo than in those treated with epoetin beta (relative risk [RR] of death, 1.39; 95% confidence interval [CI] = 1.05-1.84; P = .02). Median progression-free survival was also significantly longer in those treated with placebo (745 vs 406 days; RR of progression-free survival, 1.62; P < .001). The high final Hgb levels in the patients treated with epoetin beta in this trial (mean, 15.4 g/dL) are a possible cause of the higher number of thrombovascular events, and they may have had a negative effect on the comorbidities as well.
In the updated meta-analysis by Bohlius and colleagues of 225 randomized controlled trials in 4,307 cancer patients receiving epoetin or darbepoetin, treatment with ESPs increased the risk of thromboembolic complications (RR, 1.67; 95% CI = 1.35-2.06).[9]
Survival Impact WithESP Treatment
The updated Bohlius meta-analysis, which included the two negative trials, did not identify a definite effect of ESPs on survival.[1,9-13] The HR for overall survival was 1.08 (95%CI = 0.99-1.18).[9] Despite the inclusion of 42 trials and 8,167 patients, these data are inconclusive. None of the trials in the analysis of data on overall survival had adequate statistical power to determine whether treatment with ESPs impacts overall survival.
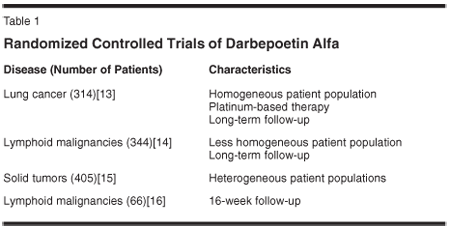
Studies have also investigated differences in survival in patients with CIA treated with the long-acting ESP darbepoetin alfa. A meta-analysis of pooled data at 16 weeks from four placebo-controlled, randomized clinical trials (Table 1) showed no differences in survival between patients treated with darbepoetin alfa and those treated with placebo. No clear signal was observed, with an HR of 1.02 (Figure 1). In one of the studies, a phase III trial in patients with lung cancer,[13] mortality was lower with darbepoetin alfa than with placebo (59% vs 69%; RR for mortality, 0.86; 95% CI = 0.38-1.01). Median survival was 46 weeks (95% CI = 39-53 weeks) in the darbepoetin alfa group and 34 weeks (95% CI = 29-39 weeks) in the placebo group.[13]
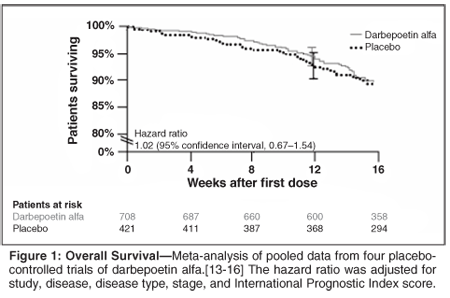
Survival Impact in Cancer: Darbepoetin Alfa Clinical Program
All studies of darbepoetin alfa worldwide were reviewed in terms of pretreatment and target Hgb levels, dosing algorithms, safety monitoring, and appropriateness of the study design for assessing survival. Because of the possible positive effect on survival with CIA treatment and the lack of a negative signal in the early studies with darbepoetin alfa, the darbepoetin alfa clinical program was designed to investigate the potential survival benefit with darbepoetin alfa in several oncology settings.
Five large, randomized controlled trials makes up a survival-focused clinical development program: one Amgen-sponsored trial (in small-cell lung cancer) and four investigator-initiated cooperative group trials (one each in non-Hodgkin's lymphoma and in head and neck cancer and two in breast cancer). The tumor types, planned and current accrual, and detectable differences from the expected control arms in these trials are shown in Table 2. Each will accrue between 600 and 1,000 patients and have the power to detect absolute differences in survival of between 7% and 11%. A meta-analysis of the five trials has an 80% power to detect a HR for survival as small as 1.15 (Figure 2); the two previously mentioned trials that showed lower survival in patients treated with ESPs had HRs of 1.31[7] and 1.39.[8] The studies are being conducted outside the United States, but their findings should be applicable in all areas.
Oncologic Drugs Advisory Committee Recommendations for Erythropoietic Therapy
Because of inconclusive data on the association of survival with ESPs to treat anemia in patients with cancer, the Oncologic Drugs Advisory Committee (ODAC) has published recommendations for the use of erythropoietic therapy in anemia. The ODAC recommends increasing the Hgb levels slowly (≥ 1 g/dL in any 2-week period), interrupting the erythropoietic therapy when the Hgb level exceeds 13 g/dL, and resuming it at 25% of the previous dose when the level has fallen to 12 g/dL or lower. Analysis of pooled data from trials of darbepoetin alfa does not show an effect on the rate of Hgb increase or in patients with an Hgb level > 13g/dL. There was no negative effect or a greater risk for thrombosis when the Hgb level was increased to 13 g/dL or higher or the level was increased by1 g/dL or more in a 2-week period.
Progression-free and overall survival were greater in patients with increases of more than 1 g/dL in a2-week period (HR for progression-free survival, 0.51 and 0.43) and in those with Hgb levels of 13 g/dL or higher (HR for progression-free survival, 0.66 and 0.56).[13-16] No obvious signal for thrombotic events was observed.
The data on survival and thrombotic risk are also confounded by the likelihood that patients with less tumor burden, less comorbid disease, and less treatment are more likely to have higher Hgb levels. Until more conclusive data are presented, the guidelines of the NCCN for managing Hgb levels in the 11 to 13 g/dL range should be followed. The ODAC also recommends that future studies consider the following:
(1) Homogeneous primary tumor type
(2) Homogeneous chemotherapy or radiotherapy regimens
(3) Design to detect clinically meaningful differences in response rate, progression-free survival, and overall survival
(4) Specified definitions of cardiovascular and thrombotic events
(5) Oversight by safety-monitoring board
(6) Determination of expression and ligand affinity of erythropoietin receptors on specific primary tumor types
(7) Analysis of clinical tissue specimens
(8) Analysis of tissue repositories representing common tumor types
Discussion
Erythropoiesis-stimulating proteins are indicated for the treatment of CIA, and their safety and efficacy have been shown in numerous published studies. Thrombotic events during treatment with these agents may be minimized by adjusting the dose to produce and maintain Hgb levels of 12 g/dL or lower and monitoring the increase in the Hgb level so that it does not exceed 1 g/dL in 2 weeks. There is concern about the potential of ESPs to increase tumor growth, but this has not yet been validated clinically and warrants prospective clinical trials.
Two studies have shown lower survival in patients treated with ESPs, but a review of the literature shows that ESPs have no clear effect on survival. The optimal design of future studies may lead to more consistent and conclusive findings. The ongoing randomized trials of darbepoetin alfa are well designed to assess survival end points, and their results are eagerly awaited.
Disclosures:
Dr. Crawford has received research funding from and has served on the advisory boards of Amgen and Ortho Biotech.
References:
1. Bohlius JF, Langensiepen S, Schwarzer G, et al: Does erythropoietin improve overall survival in the treatment of patients with malignant diseases? Results of a comprehensive analysis (abstract 709). Blood 102:203a, 2003.
2. Caro JJ, Salas M, Ward A, et al: Anemia as an independent prognostic factor for survival in patients with cancer. Cancer 91:2214-2221, 2001.
3. Grogan M, Thomas G, Melamed I, et al: The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 86:1528-1536, 1999.
4. Overgaard J, Horsman MR: Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol 6:10-21, 1996.
5. Glaser C, Millesi W, Kornek GV, et al: Impact of hemoglobin level and use of recombinant erythropoietin on efficacy of preoperative chemoradiation therapy for squamous cell carcinoma of the oral cavity and oropharynx. Int JRadiat Oncol Biol Phys 50:705-715, 2001.
6. Hardee ME, Arcasoy MO, Blackwell KL, et al: Erythropoietin biology in cancer. Clin Cancer Res 12:332-339, 2006.
7. Leyland-Jones B, Semiglazov V, Pawlicki M, et al: Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol 23:5960-5972, 2005.
8. Henke M, Laszig R, Rube C, et al: Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 362:1255-1260, 2003.
9. Bohlius J, Wilson J, Seidenfeld J, et al: Recombinant human erythropoietins and cancer patients; Updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 98:708-714, 2006.
10. Rosen FR, Haraf DJ, Kies MS, et al: Multicenter randomized phase II study of paclitaxel (1-hour infusion), fluorouracil, hydroxyurea, and concomitant twice daily radiation with or without erythropoietin for advanced head and neck cancer. Clin Cancer Res 9:1689-1697, 2003.
11. Bamias A, Aravantinos G, Kalofonos C, et al: Prevention of anemia in patients with solid tumors receiving platinum-based chemotherapy by recombinant human erythropoietin: A prospective, open label, randomized trial by the Hellenic Cooperative Oncology Group. Oncology 64:102-110, 2003.
12. Witzig TE, Silberstein PT, Loprinzi CL, et al: Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol 23:2606-2617, 2005.
13. Vansteenkiste J, Pirker R, Massuti B, et al: Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 94:1211-1220, 2002.
14. Hedenus M, Adriansson M, San Miguel, et al: Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: A randomized, double-blind, placebo-controlled study. Br J Haematol 122:394-403, 2003.
15. Hedenus M, Hansen S, Taylor K, et al: Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. Br J Haematol 119:79-86, 2002.
16. Kotasek D, Steger G, Faught W, et al: Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy: Results of a double-blind, placebo-controlled, randomised study. Eur J Cancer 39:2026-2034, 2003.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.