Erythropoietin Could Be Useful in Treating Neurologic Diseases/Trauma
KITCHAWAN, New York-Beyond its "classical" hormonal role signaling bone marrow to increase circulating red blood cells, erythropoietin (EPO) and its receptor (EPO-R) may have critical roles in the development, maintenance, protection, and repair of the brain. These roles rely on the status of the EPO and EPO-R molecules as cytokines and have been demonstrated by animal studies. Michael L. Brines, MD, PhD, senior member at the Kenneth S. Warren Institute in Kitchawan, New York, reported on these studies as well as "highly positive" results of the first human trial using recombinant human EPO to treat stroke.
KITCHAWAN, New YorkBeyond its "classical" hormonal role signaling bone marrow to increase circulating red blood cells, erythropoietin (EPO) and its receptor (EPO-R) may have critical roles in the development, maintenance, protection, and repair of the brain. These roles rely on the status of the EPO and EPO-R molecules as cytokines and have been demonstrated by animal studies. Michael L. Brines, MD, PhD, senior member at the Kenneth S. Warren Institute in Kitchawan, New York, reported on these studies as well as "highly positive" results of the first human trial using recombinant human EPO to treat stroke.
"When the erythropoietin gene was cloned in the mid 1980s and its receptor in the 1990s, it became clear immediately that these molecules were actually related to cytokines and were members of a very large family, a super family, of cytokines that had many effects in many different parts of the body," Dr. Brines said.
Dr. Brines cited an experiment with mice stripped of their EPO and EPO-R. As expected, the effect in these knock-out mice "is lethal before birth because no red blood cells were formed," Dr. Brines said. "But, in addition, if you look carefully at those embryos, one finds that the brain doesn’t develop properly." The brain and the hearts were hypoplastic and if other tissues are examined closely, they may display abnormalities as well.
Crosses Blood-Brain Barrier
While developing an animal model to evaluate the effects of erythropoietin on the feeling of well-being, researchers in Dr. Brines’s laboratory found that peripherally administered erythropoietin "was having remarkable effects on behavior. This implied that the administered erythropoietin made it through the blood-brain barrier somehow, even though it is a large molecule that’s highly glycosylated." This discovery led to a series of experiments using animal models to evaluate EPO’s potential to treat human disease.
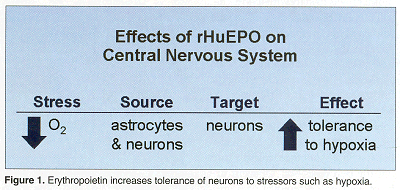
"Some of the aspects of erythropoietin activity in the brain are similar to what occurs in the periphery," Dr. Brines explained. In its hormonal roles, EPO is produced by the kidney under hypoxic stress. In vitro studies conducted nearly a decade ago revealed that astrocytes removed from the brain make erythropoietin under stress. "Subsequent studies show that one principal target is neurons and the effect is to increase tolerance to the stressor, in this case, hypoxia," he added (see Figure 1). The erythropoietin gene and its receptor make up a signaling system that influences the gene expression program in specific neurons and responds to local ischemic stress.
To determine if peripherally administered erythropoietin actually crosses the blood brain barrier, researchers injected erythropoietin systemically into rats and then tested the cerebral spinal fluid (CSF) since most substances that make it into the brain are cleared by the CSF. After a delay of 1.5 hours, the rats had levels of erythropoietin substantially above the basal level and high enough to confer neuroprotection, Dr. Brines reported. These data have been replicated
by two independent laboratories, one using sheep and the other using primates.
Brain and CNS Injuries
These findings are relevant for treating brain and CNS injuries, Dr. Brines said. Damage of brain tissue can trigger an apoptotic mechanism that leads to the death of neurons. Subsequent injury is amplified after the cell dies. Evidence for this comes from research using a stroke model in rat brains to produce a maximum penumbra that will lead to apoptosis if not treated effectively. Animals that received a saline injection at the time of the release of the stroke-producing occlusion had a large ischemic core and huge dead region formed by apoptosis. "Recombinant human erythropoietin (rHuEPO) given at the time of release of the occlusion basically salvages all of that apoptotic penumbral region, leaving the necrotic core," Dr. Brines reported. There is, however, "a time window in which erythropoietin can be administered in this model and still salvage tissue." That window occurs because apoptosis is a gene expression program that takes time to turn on and to complete. In this acute model, the time window for providing therapy that can perhaps rescue cells is within 3 hours of the injury. Some salvage still occurs at 6 hours, but by 9 hours, "you basically have missed your chance to save those cells," Dr. Brines said. For the study, the doses of rHuEPO were high5,000 units/kg. "You can give one-tenth that and still get the same effect," he said, "but below that we find that you can’t salvage those neurons."
Other Potential Uses
In a mouse model of mechanical trauma, another neurologic injury with apoptosis as a predominant feature, animals treated with rHuEPO had a reduced and smaller volume of injury than those treated with saline. As in the stroke model, there is a time window; rHuEPO can produce at least partial neuroprotective effects when administered up to 6 hours after the injury.
Dr. Brines predicted that rHuEPO would have similar effects on ischemic and traumatic injuries in the spinal cord. In a study of rabbits with ischemic injury to the spinal cord, the animals treated with saline remained paralyzed for the 48 hours the study was carried out, while "there was a remarkable recovery of neurologic function in the animals that received EPO within 1 hour." At least part of rHuEPO’s protective action against paralysis may come from altering blood flow in the spinal cord region, Dr. Brines explained.
Other reported effects of rHuEPO include anti-inflammatory action, neurotrophic effects in repair, plasticity, and synaptogenesis, and modulation of electrical excitability in the brain. The anti-inflammatory effects were demonstrated in a rat model of acute multiple sclerosis. Animals that received saline had the typical clinical course, progressing from flaccid tail, to paralysis, to death. "In animals that received EPO, the effect is not only delayed, but it’s blunted tremendously," Dr. Brines said. In contrast, animals treated with dexamethasone had no neurologic symptoms during the 18 days of treatment, but "an enormous flair" of symptoms once treatment was stopped. "So in this model, EPO was active, but it doesn’t have the rebound effect, which is a problem with some of the other treatments," Dr. Brines noted.

"EPO has potent effects on learning," Dr. Brines added, as shown in a classic learning paradigm where mice have to move through a Morris water maze. Mice treated with rHuEPO performed better than those receiving saline. Among young animals, the difference was not that great, but in older animals that tend to make more mistakes, rHu-EPO treatment raised their level of performance close to that of younger animals.
In other animal studies, rHuEPO was able to delay the onset of convulsions and seizures, with a single dose remaining effective for up to 7 days. A model of acute glaucoma demonstrated the effect of rHuEPO against retinal ischemia. "The EPO-treated animals have considerable recovery of function," Dr. Brines said, while the saline-treated animals remained blind a week later.
Clinical Implications
Dr. Brines summarized by reiterating that peripherally administered rHuEPO crosses the blood-brain barrier in sufficient amounts to initiate action and achieve results and "can be administered in advance of an injury, which is highly supportive of a concept that a breakdown of the blood-brain barrier is not essential." The effects of rHuEPO include modulation of apoptosis, inflammation, excitability, and synaptogenesis.
Studies suggest that rHuEPO may be useful in treating human disease (see Table 1, above), including stroke, trauma, epilepsy, subarachnoid hemorrhage, retinal ischemia, cognitive dysfunction, and myocardial ischemia. In addition to the reported studies with multiple sclerosis, "there are good in vivo and in vitro models for Parkinson’s disease and amyotrophic lateral sclerosis and an in vitro model for Alzheimer’s disease which is very encouraging," Dr. Brines said.
Also encouraging are the results of the first human trial with rHuEPO, a phase I/II collaborative study between Dr. Brines’s team and researchers in Germany. "The results are highly positive," Dr. Brines reported, for patients admitted within 8 hours of a documented middle cerebral artery stroke and treated with EPO for 3 days.