Evaluation and Definitive Management of Medically Inoperable Early-Stage Non-Small-Cell Lung Cancer: Part 1
Lung cancer is estimated to be the second most commonly diagnosed cancer in both men and women in 2006, and the leading cause of cancer mortality. Non-small-cell lung cancer represents the majority of such cases. Most of these patients have locally advanced disease at presentation and are not eligible for curative resection. For the minority of patients who are technically resectable at presentation, lobectomy or pneumonectomy and pathologic mediastinal nodal staging offer the best overall survival. The high rate of comorbid medical illness and poor baseline pulmonary function in this population, however, make many such early-stage patients medically inoperable. For these patients, conventional single-modality radiotherapy has been the primary definitive treatment option, as discussed in part 1 of this two-part article. Numerous retrospective reports demonstrate long-term disease-free and overall survival data that are modestly superior to that expected after observation, but both local and distant failure continue to be significant risks. Investigation of radiotherapy dose escalation is ongoing, in an effort to improve local control while maintaining minimal toxicity. Additionally, emerging evidence suggests that new modalities, such as stereotactic radiosurgery and radiofrequency ablation, may also be potentially curative treatment alternatives. These modalities will be addressed in part 2.
Lung cancer is estimated to be the second most commonly diagnosed cancer in both men and women in 2006, and the leading cause of cancer mortality. Non-small-cell lung cancer represents the majority of such cases. Most of these patients have locally advanced disease at presentation and are not eligible for curative resection. For the minority of patients who are technically resectable at presentation, lobectomy or pneumonectomy and pathologic mediastinal nodal staging offer the best overall survival. The high rate of comorbid medical illness and poor baseline pulmonary function in this population, however, make many such early-stage patients medically inoperable. For these patients, conventional single-modality radiotherapy has been the primary definitive treatment option, as discussed in part 1 of this two-part article. Numerous retrospective reports demonstrate long-term disease-free and overall survival data that are modestly superior to that expected after observation, but both local and distant failure continue to be significant risks. Investigation of radiotherapy dose escalation is ongoing, in an effort to improve local control while maintaining minimal toxicity. Additionally, emerging evidence suggests that new modalities, such as stereotactic radiosurgery and radiofrequency ablation, may also be potentially curative treatment alternatives. These modalities will be addressed in part 2.
Lung cancer is estimated to be the second most commonly diagnosed cancer in both men and women in 2006, and the leading cause of cancer mortality.[1] Non-small-cell histologies represent the majority of cases. Despite clinical investigation into screening high-risk populations, most patients have locally advanced disease at presentation and are not eligible for curative resection. For the fewer than 20% of patients with stage I or II disease, and some portion of those with stage III, surgery is the treatment of choice. The 5-year overall survival rates of patients managed with primary surgery in the modern era can be predicted as a function of clinical staging criteria: 61%, 38%, 37%, and 24% for stages IA, IB, IIA, and IIB, respectively.[2]
While surgical resection with pathologic nodal staging remains the standard of care in patients with early-stage disease, the high rate of comorbid medical illness in this population often raises concern about perioperative morbidity, postoperative pulmonary function, and long-term quality of life. An evidence-based multidisciplinary evaluation of patient age, cardiovascular health, and baseline pulmonary function can accurately predict which patients may benefit from lobectomy.
In the absence of a curative surgical option, many patients and physicians appropriately opt for either a palliative or an observational approach, but there are a substantial number of patients for whom a definitive, nonsurgical approach is appropriate. To date, definitive radiotherapy has been the most commonly employed regimen, based on data suggesting a modest survival benefit.
McGarry et al[3] analyzed the outcomes of 128 patients with stage I/II non-small-cell lung cancer (NSCLC), 47 of whom received no treatment. The median survival time with observation was 14.2 months, and the cause of death was cancer in 53% of the cases. Patients treated with radiotherapy in this series, with either palliative or curative intent, had significantly longer median survival, implying that a nonsurgical option conferred a survival benefit. Chadha et al[4] similarly reported a relatively poor median survival of 11.9 months (13.7 months for stage I and 8.4 months for stage II) for untreated early-stage NSCLC. Again, the most common cause of death was progressive disease, either local or metastatic.
The largest evaluation of the utility of radiation was conducted from a population-based registry by Wisnivesky et al.[5] The authors evaluated 4,357 patients diagnosed with stage I or II NSCLC who did not undergo surgical resection. Median survival was improved in patients treated with radiotherapy, although 5-year survival was not significantly different. Of note, the dataset did not distinguish between definitive and palliative radiation treatment courses.
For patients ineligible for curative resection, conventional single-modality radiotherapy has been the primary definitive option. Numerous retrospective reports demonstrate long-term disease-free and overall survival data that are modestly superior to that expected after observation, but both local and distant failure continue to be significant risks. Ongoing trials of dose escalation may improve local control, and the addition of systemic therapy may help to decrease metastatic failure. Additionally, emerging evidence suggests that new modalities, such as stereotactic radiosurgery and radiofrequency ablation (RFA), may offer curative treatment alternatives. These options will be discussed in the concluding part of this article, which will appear in the July issue of ONCOLOGY.
Operability
Defining "operability" in patients with lung cancer often presents a significant clinical challenge. Surgery is the treatment of choice for patients with stage I or II NSCLC. Recent studies support the use of postoperative adjuvant chemotherapy as well for treatment of early-stage NSCLC, but resection remains the primary therapeutic modality and is associated with the best long-term outcomes.[6-10] Many factors may contribute to the determination of whether an individual is suitable for lung resection. The factors that most commonly cause concern regarding morbidity or mortality following a surgical procedure are older age, the presence of significant cardiovascular risk, and the presence of underlying pulmonary disease.
Older Age
Age is increasingly a consideration in lung cancer treatment. Lung cancer generally affects an older population. Data from the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) program indicate that from 1998 to 2002, the median age at diagnosis of lung cancer was 70 years.[11] The most recent SEER data show that at initial diagnosis of lung cancer, 33.1% of patients were between ages 65 and 74, 27.9% were between ages 75 and 84, and 6.9% were age 85 years and older. As the population ages, an even larger number of patients will predictably fall into older age groups.
That said, an expanding body of evidence shows that age per se should not be a contraindication to surgery. Two recent single-institution retrospective series of pulmonary resections in octogenarians reported by Brock and colleagues (N = 68) and Port and colleagues (N = 61) noted 5-year survival rates in patients with resected stage IA NSCLC of 61% and 82%, and 30-day mortality rates of 8.8% and 1.6%, respectively.[12,13] With proper preoperative evaluation of functional status (such as the ability to perform activities of daily living), comorbidities, and cognitive function, it is clear that appropriately selected elderly patients can safely be offered curative surgical resection.[12-14]
Cardiovascular Risk
Patients with lung cancer are often at higher risk of cardiovascular disease because of shared risk factors, including cigarette smoking and older age. Since surgery for resection of lung cancer is rarely done emergently, preoperative cardiac evaluation should be possible in almost all patients. Perioperative cardiovascular risk assessment for noncardiac surgery, including thoracic surgery, has been studied extensively.
Joint evidence-based practice guidelines from the American College of Cardiology and American Heart Association have been available since 1980, with the most recent update published in 2002.[15] These guidelines make the important point that the purpose of the preoperative evaluation is not to merely grant medical clearance for surgery, but to assess the need for further preoperative testing, to plan for management of the patient's cardiac needs during and after surgery, and to guide treatment decisions.
Intrathoracic surgery falls into the category of intermediate cardiac risk, with the reported overall risk of cardiac complications usually less than 5%. Clinical predictors of increased perioperative risk of myocardial in-farction, heart failure, or death related to cardiac causes have been well described.[15] The presence of unstable coronary syndromes (acute or recent myocardial infarction or unstable angina), decompensated congestive heart failure, high-grade arrhythmias, or severe valvular disease may delay lung cancer surgery until appropriate evaluation and planning for cardiac management can be determined. Patients whose evaluations raise issues about limited life expectancy related to underlying cardiovascular disease or in whom intrathoracic surgery is deemed of unacceptable cardiovascular risk should be evaluated by appropriate specialists before a decision to deny surgery is made.
Pulmonary Disease
As with cardiovascular disease, older age and a high prevalence of cigarette smoking increase the risk for concomitant pulmonary disease. Chronic obstructive pulmonary disease (COPD) related to cigarettes is most commonly associated with lung cancer, but other lung diseases such as pulmonary fibrosis and asbestosis appear to contribute to increased risk of lung cancer as well.
Many patients with lung cancer have abnormal pulmonary function. The challenge of determining operability for these patients dates to the early days of thoracic surgery. In 1955, Gaensler and colleagues addressed the risk of respiratory failure and death related to chest surgeries in patients with severe pulmonary tuberculosis.[16] Their landmark study was the first to report surgical outcomes in relation to preoperative pulmonary physiologic measurements (vital capacity and maximal breathing capacity) and to define thresholds for operability based on such measurements.
In the 50 years that have passed since their observations, physiologic measurements have remained the cornerstone of operative risk assessment in patients undergoing lung resection. Those measurements typically used to determine resectability include absolute and percent predicted values of forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO), as well as exercise capacity. The latter is usually expressed as maximal oxygen consumption (VO2max) measured during formal cardiopulmonary exercise testing.
A number of algorithms using these measurements to identify patients who can safely undergo lung resection have been proposed.[17-21] A summary of recommendations relating to assessment of resectability from the evidence-based guidelines for lung cancer proposed by the American College of Chest Physicians is outlined in Figure 1.[22]
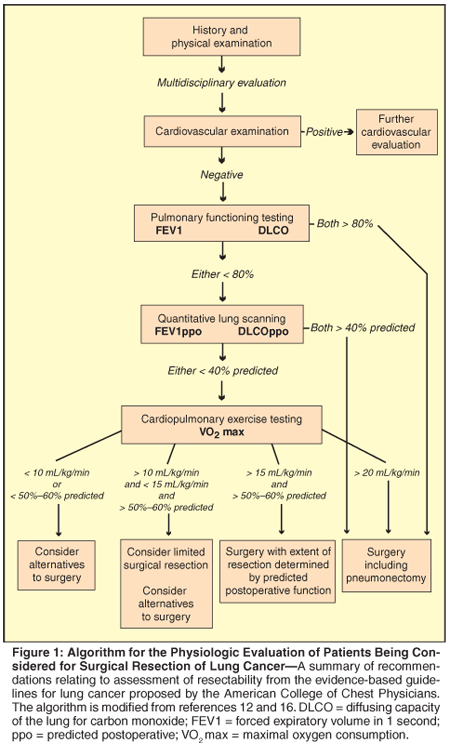
• Assessment Considerations-It is widely accepted that patients with absolute FEV1 > 2 L are candidates for pneumonectomy, and those with FEV1 > 1.5 L, candidates for lobectomy without further physiologic testing. These recommendations are based on older series done largely in men, with reported operative mortality < 5%.[17] These studies typically did not report FEV1 as a percentage of predicted normal value, but a normal FEV1 (> 80% predicted) is also accepted as a criterion for resectability.[18,22] Since cigarette smoking is so common a factor in lung cancer, obstructive airway disease is typically the accompanying pulmonary disease, with measurement of FEV1 providing a reasonable means of assessing severity.
In patients with evidence of interstitial lung disease on radiographic evaluation or in whom dyspnea is a prominent symptom, DLCO is also a useful measurement. Impairments in DLCO correlate with increased surgical morbidity as well as with worse quality of life after resection.[23,24] Patients who have normal FEV1 and DLCO (ie, both > 80% predicted) should be considered suitable candidates for resection, including pneumonectomy, without further pulmonary evaluation.
The demographics of lung cancer have changed considerably over the past several decades, with women and older persons now comprising a larger percentage of patients. Persons who are female, older, of certain ethnicities (including African or Asian descent), and who are of smaller stature will have smaller lung volumes at their normal baseline. With this consideration, using percent predicted values of FEV1 and other physiologic measurements rather than absolute values are in general more reliable in assessing lung function.
In patients who have either abnormal FEV1 or DLCO, further evaluation is necessary to determine whether resection can be performed with acceptable operative mortality and postoperative morbidity and quality of life. Predictions of postoperative values of FEV1, DLCO, and VO2max require an estimation of how much lung function will be lost with resection. The usual means of measuring "split lung" function is by radionuclide quantitative lung scanning to assess perfusion to different sides and areas of the lung in combination with measurements of FEV1, DLCO, and VO2max. Predicted postoperative FEV1 (FEV1ppo) is calculated as follows:
FEV1ppo = preoperative FEV1 X (1 - fractional contribution of lung to be resected as estimated by lung perfusion scanning)
Several studies have demonstrated reliable correlation of predicted and measured postoperative lung function using this method.[25,26] Notably, there is evidence that patients undergoing lobectomy typically have more recovery of pulmonary function (measured as both FEV1 and VO2max) within 3 to 6 months after surgery than would be predicted by split lung function prediction, so that using this method, if anything, errs on the side of patient safety.[22,27,28]
The lower limit of acceptable FEV1ppo remains controversial. Typically, FEV1ppo of 40% of the predicted normal is felt to be the minimum threshold, although some groups have suggested that 30% may also be acceptable.[29,30] Similarly, percent predicted postoperative DLCO (DLCOppo) of < 40% appears to correlate with increased perioperative complications and worsened postoperative pulmonary quality of life.[23,27,31] Patients with both FEV1ppo and DLCOppo < 40% would generally be felt to have a high risk of surgical morbidity and postoperative severe pulmonary impairment.[18,22] For some patients in this group, further physiologic assessment with cardiopulmonary exercise testing may be warranted to assess whether resection can still be an option. Alternatively, nonsurgical therapeutic modalities should be considered.
• Cardiopulmonary Exercise Testing-Exercise capacity can be estimated by simple stair climbing, or may be measured with formal cardio-pulmonary exercise testing (CPET). Stair climbing has been used for years as a measure of operability, although the number of stairs required to predict successful surgical outcome has been variably reported. Olsen and colleagues reported that to achieve successful surgical outcome a patient needed to climb at least 76 stairs for lobectomy and at least 100 steps for pneumonectomy.[32] Pollock and colleagues reported that 83 stairs would be acceptable for pneumonectomy, and correlated this amount of effort with a VO2max of 20 mL/kg/min.[33]
Formal cardiopulmonary exercise testing may be less readily available than stair climbing, but offers the ability to measure VO2max in a more standardized fashion. Both the British Thoracic Society and the American College of Chest Physician guidelines for lung cancer evaluation and treatment recommend that CPET be performed to measure VO2max if FEV1ppo and DLCOppo are < 40%.[17,22] Based on a number of studies correlating VO2max with operative risk, it is generally accepted that patients with preoperative VO2max > 20 mL/kg/min can undergo pneumonectomy without increased risk of perioperative complications, while VO2max < 15 mL/kg/min is associated with an increased risk of perioperative complications.[31,34-42] Further, patients with preoperative VO2max < 10 mL/kg/min are at very high risk of perioperative complications even with lobectomy only.[18,35,40,43] Patients with VO2max in this range will need careful evaluation before a decision regarding resectability can be made.
As with measurements of FEV1, concern has been raised about predictions based on absolute values of VO2max, as women and persons of older age and shorter stature might have normal predicted values of VO2max < 15-20 mL/kg/min. Several recent studies suggest that percent predicted VO2max is a better predictor of surgical outcome than absolute preoperative VO2max.[36,37,41,44] These studies suggest that perioperative complications are substantially increased in patients with percent predicted VO2max < 50%-60%, but that patients who have exercise capacity above this threshold can undergo surgery with reasonable safety.
Treatment Alternatives
The issue of resectability in patients whose physiologic evaluation raises concerns about perioperative risk and postoperative pulmonary compromise clearly can be very challenging. Such patients should undergo evaluation by a multidisciplinary team at a center with the necessary expertise in pulmonary medicine and thoracic surgery before a final decision regarding the feasibility of resection is made.
It should be noted that alternatives to classical lobectomy and pneumonectomy may be a consideration for individual patients. Lung-sparing surgeries such as segmentectomy and wedge resection may be reasonable in patients with severely diminished pulmonary function, and may offer such patients good long-term outcomes.[45-47] Experience in patients with severe emphysema undergoing lung volume reduction surgery has shown that carefully selected patients with very poor lung function can safely undergo thoracotomy and may have functional benefit from removal of severely emphysematous regions of lung.[48,49] In some cases, lung cancers contained within such areas can also be resected, even though the physiologic parameters outlined in Figure 1 are not met.[50,51] However, these patients should be very carefully selected, with evaluation performed at centers with specific expertise in lung cancer and lung volume reduction surgery.
The question of what constitutes acceptable risk for potentially curative surgery remains extremely difficult to answer. Treatment evaluation in patients who have substantial risk for perioperative complications and postoperative compromise of quality of life related to limited pulmonary reserve should include careful consideration of alternatives to surgery. As with other aspects of lung cancer management, decisions relating to treatment for patients with impaired lung function should involve multidisciplinary input from an experienced team of specialists in relevant disciplines, including pulmonary medicine, thoracic surgery, medical oncology, and therapeutic radiology.
Conventional Definitive Radiotherapy
Primary radiotherapy has been used as the sole treatment modality for medically inoperable, early-stage NSCLC patients for several decades,[5,52-76] and has become the standard of care in this population. Historically, there have been a wide variety of dose fractionation schemes and radiotherapy targets used, resulting in a similar variety of outcomes. Despite increased sensitivity of staging imaging and advances in treatment technology, there have been only modest improvements in reported local control and survival. Analysis of predominantly retrospective, single-institution experiences has led to improvements in radiation dose and treatment planning, and has identified areas where further advances may be made.
Nodal Irradiation
In the earliest series reporting the definitive use of radiotherapy in stage I/II tumors, relatively large treatment volumes were used, encompassing the primary tumor as well as hilar and mediastinal nodal regions.[52-54] The elective inclusion of clinically uninvolved nodal regions increases the volume of normal lung tissue within the radiation portal, increasing the risk of acute and late pulmonary toxicity.[77] The common use of computed tomography (CT) imaging, as well as the advent of 18F-fluorodeoxyglucose positron-emission tomgraphy (FDG-PET) staging, has theoretically more accurately identified areas either harboring disease or at risk, and obviated the need for these large treatment fields in many cases.
Moreover, several authors have specifically examined the utility of such treatment in terms of local, nodal, and distant failure. Krol et al[57] reviewed the outcome of 108 patients with inoperable early-stage disease treated with definitive radiation alone. In the subset of complete responders, 18% failed locally, 4% failed locally and regionally, 6% failed locally and distally, and 6% failed distally. Only 4% relapsed in the regional lymph nodes only. Hayakawa et al[55] reviewed the treatment results of 36 patients with stage I disease. In patients who did not receive elective nodal irradiation, the investigators found only a 3% isolated regional failure rate. Similarly, Slotman et al[70] noted a 6% regional nodal failure rate and Lagerwaard et al[72] reported a 0% rate after treatment with local (ie, limited to the gross tumor) radiotherapy fields.
Bradley et al[73] compared patients who received 45 to 50 Gy of elective regional lymph node irradiation with those treated at the same institution without elective regional lymph node irradiation. These investigators found a 6% regional failure rate in untreated nodal regions, and no significant difference in cause-specific or overall survival between the two groups. Finally, Sibley[63] reviewed 10 published series of definitive radiation in patients with stage I NSCLC. Treatment volumes varied from small, localized fields to comprehensive elective lymph node coverage. Analysis of long-term outcomes demonstrated that 15% of patients were long-term survivors, 25% died of intercurrent disease, 30% died from distant metastasis, and 30% died from local failure only. The researchers observed no benefit to elective lymph node irradiation. Instead, they found a local control and disease-free survival benefit associated with higher radiotherapy doses.
Taken together, this analysis of patterns of failure data has led to the conclusion that elective nodal irradiation has little role in the treatment of clinically node-negative early NSCLC.
Dose Escalation
The continued risk of local failure after radiotherapy has generated interest in determining whether increasing tumor dose would result in improved control. The standard radiation dose for definitive treatment of NSCLC, regardless of stage, has long been based on the Radiation Therapy Oncology Group (RTOG) 7301 trial.[78] This randomized dose escalation trial included patients with inoperable stage III or medically inoperable stage I or II disease, randomized to 40 Gy in a split course (20 Gy in 1 week followed by a 2-week rest and then an additional 20 Gy), or 40, 50, or 60 Gy in a continuous course delivered in 2-Gy fractions. Radiotherapy fields included the gross tumor volume as well as involved and electively included lymph nodes. The cohorts of patients who received the 50- or 60-Gy continuous-course regimen showed a decreased in-field failure rate (49% at 50 Gy, and 35% at 60 Gy) as well as increased time to failure (12 and 19 months, respectively). Since publication of this study, a minimum dose of at least 60 Gy has been considered by many to represent the standard definitive treatment dose.
Several authors have formally investigated the benefits of further dose escalation. The RTOG 9311 study is one of the largest modern prospective trials to examine doses greater than 60 Gy.[79] The authors stratified patients with stage I-III NSCLC into two groups, according to the volume of normal lung irradiated, and escalated tumor doses to 70.9 Gy, 77.4 Gy, 83.8 Gy, and 90.3 Gy in 2.1-Gy daily fractions. The lower lung-volume group, the majority of which were stage I and II patients, reached dose-limiting toxicity at the 90.3-Gy level. Acute grade 3 pneumonitis was seen in only 9%, and there were no cases of acute grade 3 esophagitis. Late toxicity, however, was significant: 13% late grade 3 or greater pulmonary toxicity including two treatment-related deaths, and 6% late grade 3 or greater esophagitis. The authors concluded that this dose level conferred an unacceptably high risk. Although they found no significant difference in overall survival or local control between the dose levels, the primary end point of the study was toxicity.
Rosenzweig et al[80] found a similar tolerated dose in their single-institution prospective dose escalation trial. They enrolled 104 patients, 28% of whom had stage I or II disease, at dose levels of 70.2 Gy, 75.6 Gy, 81 Gy, and 84 Gy, and 90 Gy. Among seven patients treated to 90 Gy, two cases of grade 3 and one of grade 5 acute pulmonary toxicity were reported; this was deemed unacceptable. Of 26 patients treated to 84 Gy, only one patient had grade 3 or greater acute pneumonitis, and two had grade 3 late pulmonary toxicity. In contrast to the lack of benefit of higher doses in the RTOG trial, however, an overall survival advantage was seen in patients treated to greater than 80 Gy.
Chen et al[76] also found a survival advantage to higher-dose radiotherapy. The authors examined the stage I and II patients enrolled in the University of Michigan dose escalation trial. Using conformal techniques, patients were treated to doses ranging from 63 to 102.9 Gy in daily fractions. On multivariate analysis, increasing radiation dose was a significant prognostic factor for overall survival, with each 1 Gy in dose escalation associated with a 3% reduction in the risk of death. The median survival for patients treated to the highest dose was 33 months, vs 27 months for those treated in the first dose escalation cohort.
Previously published results of dose escalation in the larger cohort of patients, including advanced-stage patients, had found the highest dose level to carry no significant pulmonary risk.[81] Taken together, these trials provide preliminary evidence that dose escalation over 60 Gy may convey a clinical benefit and that a moderately increased dose confers a low risk of acute and late toxicity. Similar studies have been published establishing the tolerability of dose escalation in patients with more advanced disease and with concurrent chemotherapy.[82]
It is important to note that the studies demonstrating tolerance of these doses all used validated metrics of lung dose and volume, and limited dose escalation in patients deemed at lower risk of pneumonitis. Yet even in these carefully selected patients, using the most modern treatment planning, significant toxicity was noted at the highest dose levels. The optimal dose, balancing local control and treatment-related side effects, remains to be determined and likely will be a function of individual patient pulmonary function and tumor characteristics. Additionally, incremental gains in local control and survival must be balanced against increased time on treatment in this typically elderly population. For this reason, the tolerability and improvements in survival conferred by dose escalation must be borne out in larger cohorts and in appropriate clinical trials.
Continuous- vs Split-Course Radiotherapy
A further area of interest is the value of continuous-course radiotherapy. The most common modern treatment regimen is an uninterrupted course of radiotherapy, typically 60 Gy in 30 fractions over 6 weeks. Several authors have examined split-course radiotherapy using larger fraction size to a total dose of approximately 54 Gy, also over a 6-week period but with a 2-week treatment break at weeks 3 and 4. The addition of such a treatment break has been associated with decreased acute toxicity.[52]
Several studies show no significant difference in outcome between split-course and continuous-course radiotherapy.[57,65,83] This is also supported by a prospective trial involving 273 patients randomized to receive continuous-course vs split-course treatment.[84] This study showed no significant difference in survival (10.9 months for continuous-course vs 11.6 months for split-course radiotherapy), although increased morbidity was noted with the continuous-course regimen.
In contrast, Haffty et al[52] noted improved survival (45% vs 12% at 5 years) and improved local control with continuous-course radiotherapy. This was also the finding of an RTOG prospective randomized trial that involved 551 patients (with early- and advanced-stage disease).[78] Patients with earlier-stage disease showed improved local control with continuous-course vs split-course radiotherapy (27% vs 38% intrathoracic failure rate).
Despite the apparently inconclusive evidence, continuous radiotherapy has become the most common prescribed course. Given the low reported toxicity of limited-field pulmonary radiation in patients with early-stage disease, treatment breaks are infrequently necessary, and with concern for tumor repopulation during therapy, overall treatment time is typically minimized.
Fractionated Radiotherapy
Both hyper- and hypofractionated, accelerated radiotherapy have been investigated in this setting to determine whether there is any additional benefit or decreased toxicity. The Netherlands Cancer Institute dose escalation trial[85] investigated increasing radiation doses using 2.25-Gy fractions. At doses higher than 67.5 Gy, twice-daily treatment was used to prevent further prolongation of treatment time. Patients were stratified by mean lung dose, and the dose was escalated until limiting toxicity was reached. For the two lowest-volume strata, which included the vast majority of patients with early-stage disease, doses of 87.8 and 81 Gy were reached. The authors concluded that the accelerated fractionation regimen was tolerated without excess toxicity.
Continuous hyperfractionated accelerated radiotherapy (CHART) is a strategy of three-times-daily radiotherapy to 54 Gy in 12 total treatment days. This was tested against standard daily radiotherapy in a randomized trial of 563 NSCLC patients, 169 of whom had early-stage disease.[86] CHART improved overall survival at 2 years-29% vs 20% for standard radiotherapy. Acute esophagitis, however, was also significantly increased. Acute pneumonitis was not increased in the CHART arm (10% vs 19% for standard treatment), but a trend toward increased late pneumonitis was seen.
Hypofractionated radiotherapy has also been investigated, in an effort to deliver a higher dose per fraction in a significantly shorter treatment time to minimize tumor cell repopulation. The use of large fraction sizes produces a theoretical risk of increasing late toxicity. The clinical experience, although limited, seems to demonstrate that such regimens are both well tolerated and effective. Slotman et al[70] treated 31 stage I patients to 48 Gy in 12 fractions. The overall survival rate at 3 years was 42%, and the researchers found no clinically significant late pneumonitis. Cheung et al[68] used the same accelerated hypofractionated treatment course in 33 patients with early-stage disease, and reported a 3-year overall survival rate of 43%. Significant pneumonitis, both acute and chronic, was noted in less than 10% of patients. Overall, both trials demonstrate long-term survival comparable to that seen in more conventionally fractionated trials, with acceptable toxicity.
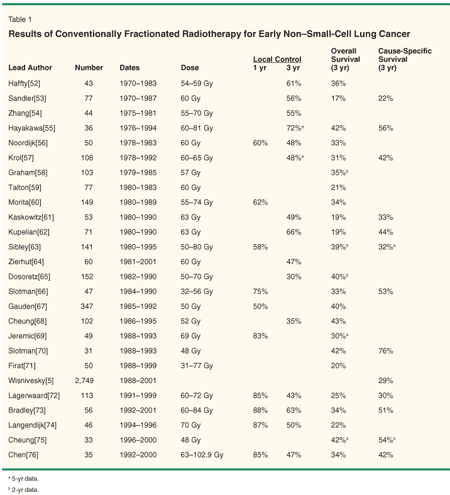
The clinical results of definitive, fractionated radiotherapy are presented in Table 1.[5,52-76] Typically, the patients included in these trials were felt to be medically ineligible for surgery, or refused surgery. The median age ranges from 57 to 74 years. A heterogeneous group of tumor stages are represented, including clinically node-positive patients. In modern series with appropriate staging and tumor doses of 60 Gy or greater, the reported short-term (ie, < 1 year) local control rate ranges from 60% to 90%; at 3 years, the reported range is 40% to 70%. Overall survival in these studies is low-20% to 40% at 3 years-likely reflecting the advanced age and comorbid illness inherent in this population. Cause-specific survival is the most appropriate end point in assessing the efficacy of treatment, given a high risk of intercurrent death, and ranges from 20% to 50% at 3 years. Significant variables affecting local control and survival include radiation dose[63,80] and tumor size.[65]
• Toxicity-The reported toxicity in these series is low. In contrast to the treatment of locally advanced disease, radiotherapy portals for patients with medically inoperable early-stage NSCLC are typically smaller, encompassing the tumor with a small margin to account for microscopic tumor extension, respiratory motion, and patient set-up error. While esophagitis is the acute dose-limiting toxicity in stage III disease, this would not be expected in patients with stage I or II disease in the absence of elective mediastinal lymph node irradiation. The most clinically concerning intermediate and late toxicity related to treatment of advanced-stage disease is pneumonitis, the risk of which is known to be a function of the volume of lung irradiated. As expected, given the typically small treatment fields used for early-stage disease, this risk has been demonstrated to be low with conventional treatment to small primary tumors.
Clinically significant (RTOG/European Organization for Research and Treatment of Cancer [EORTC] common toxicity scoring criteria grade 3 or above) pneumonitis was reported in less than 10% of patients in the listed series. Authors who specifically did not treat clinically uninvolved nodal regions reported rates of 5% or less. This low expectation of treatment-related side effects is appropriate for this patient population, given that the majority of patients are not at imminent risk of symptoms from their disease and typically have significant comorbid illnesses.
• Conclusions-Primary radiotherapy has demonstrated efficacy in the definitive treatment of medically inoperable NSCLC. While many medically inoperable patients have significant comorbid illnesses, a significant proportion will die of progressive lung cancer without definitive treatment.[3] Survival following treatment is superior to that seen with observation[3] in multiple series with adequate follow-up, making this the standard treatment for patients not eligible for curative surgery. Even in modern radiotherapy series, however, the risk of both local and distant failure remains significant. In terms of the former, results of recent and current ongoing dose escalation trials hold promise for improving patient outcomes. Given that this patient population is typically elderly, with significant comorbid medical illness (and generally asymptomatic from their disease), advances in definitive treatment must be undertaken with care to maintain the expected low side-effect profile, and with the understanding that prolongation of treatment time may not be appropriate for some patients.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2006. CA Cancer J Clin 56:106-130, 2006.
2. Mountain CF: The international system for staging lung cancer. Semin Surg Oncol 18:106-115, 2000.
3. McGarry RC, Song G, des Rosiers P, et al: Observation-only management of early stage, medically inoperable lung cancer: Poor outcome. Chest 121:1155-1158, 2002.
4. Chadha AS, Ganti AK, Sohi JS, et al: Survival in untreated early stage non-small cell lung cancer. Anticancer Res 25:3517-3520, 2005.
5. Wisnivesky JP, Bonomi M, Henschke C, et al: Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 128:1461-1467, 2005.
6. Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351-360, 2004.
7. Blum RH: Adjuvant chemotherapy for lung cancer-a new standard of care. N Engl J Med 350:404-405, 2004.
8. Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352:2589-2597, 2005.
9. Kato H, Ichinose Y, Ohta M, et al: A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713-1721, 2004.
10. Pisters KM: Adjuvant chemotherapy for non-small-cell lung cancer-the smoke clears. N Engl J Med 352:2640-2642, 2005.
11. Ries LAG, Eisner MP, Kosary CL, et al: SEER Cancer Statistics Review, 1975-2002. National Cancer Institute, Bethesda, Md, 2005.
12. Brock MV, Kim MP, Hooker CM, et al: Pulmonary resection in octogenarians with stage I nonsmall cell lung cancer: A 22-year experience. Ann Thorac Surg 77:271-277, 2004.
13. Port JL, Kent M, Korst RJ, et al: Surgical resection for lung cancer in the octogenarian. Chest 126:733-738, 2004.
14. Fukuse T, Satoda N, Hijiya K, et al: Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest 127:886-891, 2005.
15. Eagle KA, Berger PB, Calkins H, et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery-executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542-553, 2002.
16. Gaensler EA, Cugell DW, Lindgren I, et al: The role of pulmonary insufficiency in mortality and invalidism following surgery for pulmonary tuberculosis. J Thorac Surg 29:163-187, 1955.
17. BTS guidelines: Guidelines on the selection of patients with lung cancer for surgery. Thorax 56:89-108, 2001.
18. Wyser C, Stulz P, Soler M, et al: Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 159:1450-1456, 1999.
19. Tanoue LT: Preoperative evaluation of the high-risk surgical patient for lung cancer resection. Semin Respir Crit Care Med 21:421-432, 2000.
20. Tanoue LT, Ponn RB: Therapy for stage I and stage II non-small cell lung cancer. Clin Chest Med 23:173-190, 2002.
21. Datta D, Lahiri B: Preoperative evaluation of patients undergoing lung resection surgery. Chest 123:2096-2103, 2003.
22. Beckles MA, Spiro SG, Colice GL, et al: The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest 123:105S-114S, 2003.
23. Ferguson MK, Little L, Rizzo L, et al: Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 96:894-900, 1988.
24. Handy JR Jr, Asaph JW, Skokan L, et al: What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 122:21-30, 2002.
25. Kristersson S, Lindell SE, Svanberg L: Prediction of pulmonary function loss due to pneumonectomy using 133 Xe-radiospirometry. Chest 62:694-698, 1972.
26. Wernly JA, DeMeester TR, Kirchner PT, et al: Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 80:535-543, 1980.
27. Corris PA, Ellis DA, Hawkins T, et al: Use of radionuclide scanning in the preoperative estimation of pulmonary function after pneumonectomy. Thorax 42:285-291, 1987.
28. Nezu K, Kushibe K, Tojo T, et al: Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 113:1511-1516, 1998.
29. Gass GD, Olsen GN: Preoperative pulmonary function testing to predict postoperative morbidity and mortality. Chest 89:127-135, 1986.
30. Nakahara K, Ohno K, Hashimoto J, et al: Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg 46:549-552, 1988.
31. Markos J, Mullan BP, Hillman DR, et al: Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 139:902-910, 1989.
32. Olsen GN, Bolton JW, Weiman DS, et al: Stair climbing as an exercise test to predict the postoperative complications of lung resection. Two years' experience. Chest 99:587-590, 1991.
33. Pollock M, Roa J, Benditt J, et al: Estimation of ventilatory reserve by stair climbing. A study in patients with chronic airflow obstruction. Chest 104:1378-1383, 1993.
34. Pate P, Tenholder MF, Griffin JP, et al: Preoperative assessment of the high-risk patient for lung resection. Ann Thorac Surg 61:1494-1500, 1996.
35. Bechard D, Wetstein L: Assessment of exercise oxygen consumption as preoperative criterion for lung resection. Ann Thorac Surg 44:344-349, 1987.
36. Richter LK, Svendsen UG, Milman N, et al: Exercise testing in the preoperative evaluation of patients with bronchogenic carcinoma. Eur Respir J 10:1559-1565, 1997.
37. Morice RC, Peters EJ, Ryan MB, et al: Exercise testing in the evaluation of patients at high risk for complications from lung resection. Chest 101:356-361, 1992.
38. Bolliger CT, Soler M, Stulz P, et al: Evaluation of high-risk lung resection candidates: pulmonary haemodynamics versus exercise testing. A series of five patients. Respiration 61:181-186, 1994.
39. Walsh GL, Morice RC, Putnam JB Jr, et al: Resection of lung cancer is justified in high-risk patients selected by exercise oxygen consumption. Ann Thorac Surg 58:704-710, 1994.
40. Brutsche MH, Spiliopoulos A, Bolliger CT, et al: Exercise capacity and extent of resection as predictors of surgical risk in lung cancer. Eur Respir J 15:828-832, 2000.
41. Bolliger CT, Jordan P, Soler M, et al: Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med 151:1472-1480, 1995.
42. Smith TP, Kinasewitz GT, Tucker WY, et al: Exercise capacity as a predictor of post-thoracotomy morbidity. Am Rev Respir Dis 129:730-734, 1984.
43. Holden DA, Rice TW, Stelmach K, et al: Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest 102:1774-1779, 1992.
44. Win T, Jackson A, Sharples L, et al: Cardiopulmonary exercise tests and lung cancer surgical outcome. Chest 127:1159-1165, 2005.
45. Martini N, Bains MS, Burt ME, et al: Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 109:120-129, 1995.
46. Ginsberg RJ, Rubinstein LV: Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60:615-622, 1995.
47. Smythe WR: Treatment of stage I non-small cell lung carcinoma. Chest 123:181S-187S, 2003.
48. Ware JH: The National Emphysema Treatment Trial-how strong is the evidence? N Engl J Med 348:2055-2056, 2003.
49. Russi EW, Bloch KE, Weder W: Lung volume reduction surgery: What can we learn from the National Emphysema Treatment Trial? Eur Respir J 22:571-573, 2003.
50. Ojo TC, Martinez F, Paine R III, et al: Lung volume reduction surgery alters management of pulmonary nodules in patients with severe COPD. Chest 112:1494-1500, 1997.
51. McKenna RJ Jr, Fischel RJ, Brenner M, et al: Combined operations for lung volume reduction surgery and lung cancer. Chest 110:885-888, 1996.
52. Haffty BG, Goldberg NB, Gerstley J, et al: Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 15:69-73, 1988.
53. Sandler HM, Curran WJ Jr, Turrisi AT III: The influence of tumor size and pre-treatment staging on outcome following radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 19:9-13, 1990.
54. Zhang HX, Yin WB, Zhang LJ, et al: Curative radiotherapy of early operable non-small cell lung cancer. Radiother Oncol 14:89-94, 1989.
55. Hayakawa K, Mitsuhashi N, Saito Y, et al: Limited field irradiation for medically inoperable patients with peripheral stage I non-small cell lung cancer. Lung Cancer 26:137-142, 1999.
56. Noordijk EM, vd Poest Clement E, Hermans J, et al: Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 13:83-89, 1988.
57. Krol AD, Aussems P, Noordijk EM, et al: Local irradiation alone for peripheral stage I lung cancer: Could we omit the elective regional nodal irradiation? Int J Radiat Oncol Biol Phys 34:297-302, 1996.
58. Graham MV, Purdy JA, Emami B, et al: Preliminary results of a prospective trial using three dimensional radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 33:993-1000, 1995.
59. Talton BM, Constable WC, Kersh CR: Curative radiotherapy in non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 19:15-21, 1990.
60. Morita K, Fuwa N, Suzuki Y, et al: Radical radiotherapy for medically inoperable non-small cell lung cancer in clinical stage I: A retrospective analysis of 149 patients. Radiother Oncol 42:31-36, 1997.
61. Kaskowitz L, Graham MV, Emami B, et al: Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 27:517-523, 1993.
62. Kupelian PA, Komaki R, Allen P: Prognostic factors in the treatment of node-negative nonsmall cell lung carcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys 36:607-613, 1996.
63. Sibley GS: Radiotherapy for patients with medically inoperable stage I nonsmall cell lung carcinoma: smaller volumes and higher doses-a review. Cancer 82:433-438, 1998.
64. Zierhut D, Bettscheider C, Schubert K, et al: Radiation therapy of stage I and II non-small cell lung cancer (NSCLC). Lung Cancer 34(suppl 3):S39-S43, 2001.
65. Dosoretz DE, Katin MJ, Blitzer PH, et al: Radiation therapy in the management of medically inoperable carcinoma of the lung: Results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys 24:3-9, 1992.
66. Slotman BJ, Njo KH, Karim AB: Curative radiotherapy for technically operable stage I nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 29:33-37, 1994.
67. Gauden S, Ramsay J, Tripcony L: The curative treatment by radiotherapy alone of stage I non-small cell carcinoma of the lung. Chest 108:1278-1282, 1995.
68. Cheung PC, Yeung LT, Basrur V, et al: Accelerated hypofractionation for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 54:1014-1023, 2002.
69. Jeremic B, Shibamoto Y, Acimovic L, et al: Hyperfractionated radiotherapy alone for clinical stage I nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 38:521-525, 1997.
70. Slotman BJ, Antonisse IE, Njo KH: Limited field irradiation in early stage (T1-2N0) non-small cell lung cancer. Radiother Oncol 41:41-44, 1996.
71. Firat S, Bousamra M, Gore E, Byhardt RW: Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 52:1047-1057, 2002.
72. Lagerwaard FJ, Senan S, van Meerbeeck JP, et al: Has 3-D conformal radiotherapy (3D CRT) improved the local tumour control for stage I non-small cell lung cancer? Radiother Oncol 63:151-157, 2002.
73. Bradley JD, Wahab S, Lockett MA, et al: Elective nodal failures are uncommon in medically inoperable patients with stage I non-small-cell lung carcinoma treated with limited radiotherapy fields. Int J Radiat Oncol Biol Phys 56:342-347, 2003.
74. Langendijk JA, Aaronson NK, de Jong JM, et al: Quality of life after curative radiotherapy in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 53:847-853, 2002.
75. Cheung PC, Mackillop WJ, Dixon P, et al: Involved-field radiotherapy alone for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 48:703-710, 2000.
76. Chen M, Hayman JA, Ten Haken RK, et al: Long-term results of high-dose conformal radiotherapy for patients with medically inoperable T1-3N0 non-small-cell lung cancer: Is low incidence of regional failure due to incidental nodal irradiation? Int J Radiat Oncol Biol Phys 64:120-126, 2005.
77. Graham MV, Purdy JA, Emami B, et al: Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45:323-329, 1999.
78. Perez CA, Pajak TF, Rubin P, et al: Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 59:1874-1881, 1987.
79. Bradley J, Graham MV, Winter K, et al: Toxicity and outcome results of RTOG 9311: A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 61:318-328, 2005.
80. Rosenzweig KE, Fox JL, Yorke E, et al: Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer 103:2118-2127, 2005.
81. Narayan S, Henning GT, Ten Haken RK, et al: Results following treatment to doses of 92.4 or 102.9 Gy on a phase I dose escalation study for non-small cell lung cancer. Lung Cancer 44:79-88, 2004.
82. Socinski MA, Morris DE, Halle JS,
et al: Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: A dose-escalation phase I trial. J Clin Oncol 22:4341-4350,
2004.
83. Lee RE, Carr DT, Childs DS Jr: Comparison of split-course radiation therapy and continuous radiation therapy for unresectable bronchogenic carcinoma: 5 year results. AJR Am J Roentgenol 126:116-122, 1976.
84. Routh A, Hickman BT, Khansur T: Report of a prospective trial-split course versus conventional radiotherapy in the treatment of non small cell lung cancer. Radiat Med 13:115-119, 1995.
85. Belderbos JS, De JK, Heemsbergen WD, et al: First results of a phase I/II dose escalation trial in non-small cell lung cancer using three-dimensional conformal radiotherapy. Radiother Oncol 66:119-126, 2003.
86. Saunders M, Dische S, Barrett A, et al: Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial. CHART steering committee. Radiother Oncol 52:137-148, 1999.