Every-3-Week Schedule of Irinotecan Plus Capecitabine Achieves Better Results Than Weekly Dosing as First-Line Therapy
This special supplement to Oncology News International includes 28 reportswith updated information on clinical trials investigating capecitabine and other agents inthe treatment of advanced colorectal and breast cancers, and other solid tumors.The reports summarize selected presentations from the 39th Annual Meeting of theAmerican Society of Clinical Oncology (ASCO) and related educational symposiaheld in conjunction with ASCO.
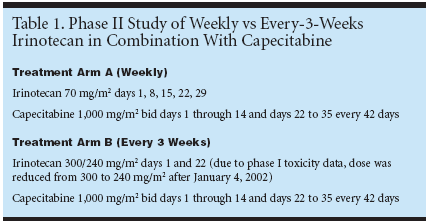
BERN, Switzerland-A study ofcapecitabine (Xeloda) and two differentschedules of administration of irinotecan(CPT-11, Camptosar) as first-line therapyfor patients with metastatic colorectalcancer found that a 3-week scheduleachieved better response than a 1-weekschedule, with reduced toxicity. The resultswere reported by Markus Borner,MD, associate professor, Institute of MedicalOncology, Inselspital, Bern, Switzerland(ASCO abstract 1068).Adding irinotecan to fluoropyrimidinesis a new standard in the treatmentof metastatic colorectal cancer. Accordingto Dr. Borner, "Well tolerated combinationregimens have used irinotecan incombination with infusional fluorouracil(5-FU) plus leucovorin. Oral capecitabinemight be more convenient than infusionalfluorouracil. Furthermore, preliminarystudy results suggest that thecombination of capecitabine and irinotecanis feasible. However, irinotecan canbe administered in different schedules andit is not clear which schedule is the mostadvantageous in combination withcapecitabine."Objectives and End PointThe primary objective of the study wasto compare the efficacy of different irinotecanschedules in combination withcapecitabine in chemotherapy-naive metastaticcolorectal cancer patients. The mainend point of this study was the objectivetumor response rate. The secondary objectiveswere to assess toxicity, time totreatment failure, time to progression, andoverall survival associated with the twodifferent schedules."We performed an exploratory phaseII study comparing weekly irinotecan to3-weekly irinotecan in combination withcapecitabine," Dr. Borner explained (Table1). In treatment arm A, irinotecan wasadministered every week at a dose of 70mg/m2 on days 1, 8, 15, 22, and 29. Intreatment arm B, irinotecan was givenevery 3 weeks at a dose of 300/240 mg/m2on day 1 and day 22. Both arms receivedcapecitabine 1,000 mg/m2 twice daily ondays 1 to 14 and days 22 to 35 every 42days. Because of newly available phase Itoxicity data, the irinotecan dose in treatmentarm B was reduced from 300 to 240mg/m2 after January 4, 2002.
To be eligible for the study, patientsmust have: nonresectable advanced ormetastatic colorectal cancer, no prior chemotherapyfor advanced or metastatic cancer,good performance status (WorldHealth Organization 0/1), and measurabledisease. The two arms were well balanced.Response and ToxicitiesThe objective response rates as assessedby investigators were 29% for arm A, and43% for arm B. Median time to progressionwas 6.9 months for arm A, and 9.3months for arm B. The median overallsurvival was 14.7 months in arm A andnot reached yet in arm B.The most important toxicities weregrade 3/4 diarrhea (32% in arm A vs 19%in arm B) and grade 2/3 alopecia (21% vs65%). Other grade 3/4 toxicities were rare(less than 5%).In summarizing the results of the study,Dr. Borner said, "The combination ofirinotecan plus capecitabine is active inthe treatment of first-line metastatic colorectalcancer. Considering the favorabletoxicity, time to progression, and patientconvenience, in addition to the main studyend point of objective tumor responserate, the every-3-week schedule of capecitabineplus irinotecan will be used for furthercomparative studies."