Evolution of the Concept of Focal Therapy for Prostate Cancer
In this review we focus on the recent evolution of the concept of focal therapy and the potential applications of this management approach within an array of options currently available for patients with localized prostate cancer.
The landscape of prostate cancer has been rapidly evolving, and technological advances in imaging and biopsy tools offer novel approaches to focal therapy. In this dynamic environment, the role of focal therapy for prostate cancer is being shaped both by advances in technology and by reconsidering the epidemiological and outcomes data for available treatments. Here we focus on the evolution of the concept of focal therapy and its potential roles in the management of prostate cancer.
Introduction
Focal therapy for prostate cancer (PCa) is a management concept whereby active therapy is delivered to malignant portion(s) of the gland, eradicating the known and targeted cancer while sparing unaffected tissue and reducing the morbidity of treatment.[1] Whole-gland therapies such as radical prostatectomy and radiation carry a significant burden of side effects, and a concern regarding active surveillance is that it potentially misses the window of opportunity for active treatment and cure. In contrast, focal therapy-which combines active treatment of the identified clinically significant disease with active surveillance of the remaining unaffected prostate tissue-offers an intermediate means of active management of PCa with potentially better-preserved quality of life for the patient.
The landscape of prostate cancer management has been rapidly evolving, with technological advances in imaging and biopsy techniques offering novel tools for focal therapy approaches.[2,3] In this dynamic environment, the role of focal therapy is still being shaped, both by the rapid introduction of new technology and through reconsideration of the evidence regarding the epidemiology of prostate cancer and available treatment options.
Here we will focus on the recent evolution of the concept of focal therapy and the potential applications of this management approach within an array of options currently available for patients with localized PCa.
Focal Therapy as the Lesser Evil
The contemporary practice of overtreatment of low-risk PCa is well documented.[4,5] Today, most men diagnosed with PCa undergo whole-gland treatment, which in some patients will be associated with significant side effects that reduce their quality of life.[6] Importantly, a recent study could not confirm an overall or cancer-specific mortality benefit for radical prostatectomy over watchful waiting in men with low-risk disease.[4] Undoubtedly, however, active intervention puts the patients at risk for potentially severe complications.
TABLE

Summary of the Available Outcomes of Focal Therapy in the Published Literature
In this setting, focal therapy, with its favorable side effects profile, may be a lesser evil. Preserving a man’s quality of life by avoiding the side effects of whole-gland treatment[7] is a significant consideration in many patients (especially those with low-risk disease), and focal therapy may be an excellent active-treatment option for prostate cancer. In fact, the morbidity of focal therapy approaches has been demonstrated to be quite favorable in several reports.[8-10] Reported potency rates are excellent, and incontinence and bowel-related side effects are extremely rare, whereas these side effects represent a considerable quality-of-life burden associated with whole-gland therapies.[7] (See the Table for a summary of currently available published outcomes of focal therapy, including oncological outcomes and complications.)
Salvaging Active Surveillance
Typically, men on active surveillance protocols harbor low-risk prostate cancer. Progression of disease on active surveillance, understaging of the disease in the initial evaluation, or simply the psychological difficulty that the surveillance strategy poses for some patients most often leads to eventual whole-gland treatment.[11] This transition from active surveillance to whole-gland therapy takes the least invasive approach and transforms it into the standard-template management of cancer, with its potential treatment-related side effects.
In this setting of preselected patients deemed suitable for the least aggressive approach-ie, active surveillance-focal therapy could be integrated seamlessly into a continuum of minimally invasive management of PCa.[12] In other words, if during active surveillance a clinically significant disease focus is identified (eg, by a Gleason score of 7, increased tumor volume, more positive biopsy cores) that “reclassifies” the patient and thereby deems him unfit for active surveillance, this lesion could be actively treated with focal therapy, eliminating the malignant focus and thus “requalifying” the patient for active surveillance. In fact, focal therapy necessarily includes elements of active surveillance, since the multifocal nature of PCa requires that the untreated portion(s) of the gland be monitored.
This approach is novel for PCa management, since we typically rely on the characteristics of PCa at the time of diagnosis while ignoring the dynamics of PCa’s natural history. Perhaps, however, this dogma should be reconsidered. Are the initial characteristics of the disease on presentation more important than staging after focal treatment? Once the focus of concerning disease (ie, the index lesion with a potentially aggressive natural history) has been eliminated, the cancer’s drive for progression has been effectively mitigated.[13]
Expanding the Indications
The evidence of overtreatment is compelling. While in patients with minimal-volume low-risk disease the benefits of any treatment may not be evident, making the right treatment choice becomes paramount in patients with intermediate- and high-risk disease, for whom the risks of disease progression and PCa-related mortality are significant.[4] Given the long natural history of PCa, even in intermediate- and high-risk patients,[14] preservation of a good quality of life is still an important factor that should be carefully weighed when considering management options.
There are no data to support the inferiority of ablative approaches in eliminating cancer cells, as compared to surgical extirpation or radiation. Moreover, the use of ablative approaches may exploit an additional antineoplastic tool-the immune system-by exposing tumor-specific antigens.[15,16] Thus, if a focal therapy approach is technically feasible, that is, if the lesions are amenable to complete ablation with preservation of healthy tissue, it may need to be considered among the management options for intermediate- and high-risk localized disease. In these patients, multimodality approaches may be required in the same manner as adjuvant treatments are employed in combination with surgery and radiation.
In addition, it has been suggested that the metastatic potential and lethality of prostate cancer may be linked to a specific clone of cancer cells,[13] identified as the index lesion-typically the largest focus of cancer with the most adverse pathological features. The evidence in the literature suggests that the index lesion is driving the natural history of prostate cancer, whereas satellite foci play little to no role in disease progression. Hence, specifically targeting the aggressive index lesion would theoretically enable us to control the malignant potential of the disease without turning to more aggressive and morbid management modalities. Utilization of focal therapy in this setting would not preclude future more aggressive treatments if these became necessary.
There is currently a drive toward expanding the criteria of patient eligibility for focal therapy. To establish focal therapy as an alternative to radical prostatectomy or radiation for select patients, the inclusion criteria need to be expanded, with an aim of recruiting intermediate- and high-risk patients for focal therapy trials to confirm the validity of the focal approach from the oncological standpoint.
Focal Therapy as a Downstaging Approach
Currently, strict criteria apply to candidates for active surveillance.[17] As a result, many men with localized, low-risk disease would not qualify[18] (based on number of positive cores, extent of cancer in a core, or prostate-specific antigen [PSA]-related measures) and would therefore resort to whole-gland treatment. In this scenario, focal therapy potentially could be used to downstage PCa, by eliminating malignant foci and reducing the burden of clinically significant disease, potentially enabling some of these patients to display the disease characteristics that would permit enrollment in active surveillance protocols. Similarly, if a patient did not meet the criteria for active surveillance because of a lesion with a Gleason score of 7 but would otherwise be eligible, this criterion could be addressed with focal therapy, effectively extending eligibility for active surveillance to select men with intermediate-risk PCa.
Integration of Focal Therapy and Active Surveillance
Focal therapy and active surveillance for prostate cancer are, in our opinion, two arms of a single management strategy for PCa. Accurate candidate selection using refined diagnostic tools (including advanced biopsy techniques and modern imaging studies) is paramount to ensuring the success of both. Following focal therapy, the untreated portion of the gland is placed, in effect, on active surveillance. Patients who are unable to continue on active surveillance protocols, those subsequently desiring active treatment, or those who have had their cancers upstaged could be offered focal therapy, if feasible. This would enable patients to avoid or further delay radical whole-gland approaches that they initially opted against by choosing active surveillance. Moreover, focal therapy may enable the disease to be downstaged so that it might meet the criteria for an active surveillance protocol, thus enabling more patients to benefit from minimally invasive management options and to avoid radical treatment.
In many ways, focal therapy and active surveillance are inter-related. Since these management options require accurate staging of the disease, we believe that they should use the same currently available diagnostic and staging tools, to permit accurate characterization and definition of the extent of disease. Modern diagnostic tools, such as multi-parametric magnetic resonance imaging (MRI) and advanced biopsy techniques (eg, transperineal template-guided mapping biopsy), should be thoughtfully implemented in the protocols of both focal therapy and active surveillance, to optimize these management options and tailor them to the characteristics of PCa in the individual patient.
Patient Selection
Given the wide array of potential uses of active surveillance and focal therapy, appropriate candidate selection is paramount. In light of the constant and rapid evolution of the concepts of focal therapy and active surveillance, along with the extension of their potential uses to a broader patient pool, candidate evaluation remains among the top concerns. Another difficulty with defining selection criteria for focal therapy is that focal therapy is not one approach but rather comprises a multitude of techniques that could be tailored to the individual patient.
These issues were illustrated in a recent review by Abern et al[12] of modern selection criteria for focal therapy; the authors summarized the available data and demonstrated that no standard widely accepted criteria exist, and that the available expert panel guidelines are not in uniform agreement. Similarly, active surveillance eligibility criteria vary between the published series.[17] Although both focal therapy and active surveillance pose similar challenges in candidate evaluation-namely the need to precisely characterize the disease and reliably exclude aggressive features of PCa, most of the currently available protocols rely on suboptimal detection and staging tools. In fact, imaging studies have only recently been proposed as part of focal therapy evaluation protocols,[12] whereas active surveillance criteria have yet to employ imaging modalities.[17] Moreover, while the focal therapy selection criteria have included extensive biopsy schemes, such as transperineal mapping biopsy, active surveillance protocols continue to rely on a routine office-based 10- to 12-core extended biopsy.
With the introduction and further assessment of modern techniques, including imaging modalities and advanced biopsy approaches, we believe that novel tools could be readily utilized to improve the accuracy and reliability of candidate evaluation for both non-radical management options.
Designing a Focal Therapy Clinical Trial
Focal therapy as an approach requires rigorous clinical trials to establish its role and indications in the modern landscape of prostate cancer. High-quality evidence is needed for the introduction of focal therapy into the professional guidelines. However, it is challenging to design a clinical trial in such a manner that its results would be clinically relevant and timely.
One of the main questions that arise while designing a trial is what the primary outcome should be. The optimal outcome for any new treatment strategy would be an overall survival benefit, compared with the standard of care. The goal would be to demonstrate noninferiority in survival while improving quality of life by-in this case by diminishing the complications associated with whole-gland therapy. For several reasons, we believe that this is not feasible in our current environment. First, such a trial would require a long follow-up period and a large number of enrolled patients, and so would need significant financial and logistical support. Second, because focal therapy itself encompasses several approaches, the results would likely not be applicable to all focal therapy strategies and treatment schemes. Third, the need for long follow-up would mean that the results of such a trial would not be available until 10 to 15 years after the end of enrollment, and thus would almost certainly be doomed to be untimely and obsolete, given the rapid advances in the field (encompassing new devices, new drugs, and other discoveries).
On an optimistic note, however, the lack of focal therapy trials is evident, leaving room for smaller, less ambitious endeavors, such as trials designed with physiologic functional outcomes (erectile function, urinary control, and other quality-of-life measures) as the primary endpoints. Such trials would need shorter follow-up and fewer patients enrolled. Similarly, although there are no defined surrogates for oncologic control in focal therapy (as PSA recurrence is a surrogate for control following radical treatment), a trial could focus on mid-term (3 to 5 years) oncologic outcomes.
Another crucial point for trial design lies in the selection of the control group, that is, the standard-of-care management to which focal therapy is to be compared. There are several options for this control treatment, and the appropriate control for focal therapy would be defined by the selection criteria for the given focal approach. For example, if a particular focal approach is investigated for low-risk patients, then active surveillance would represent an appropriate control arm. If, however, focal therapy indications are expanded to include intermediate-risk disease, then a radical treatment option would be a more suitable option for a control arm. For one-arm trials, a comparison to historical data could be used when the historical estimate is accurate and the cohort is comparable to the patient population to be enrolled for focal therapy.
Rigorous clinical trials are necessary for generating hard evidence to support the use of any novel treatment. To date, such data are lacking for focal therapy, although a number of more rigorous trials are under way. We believe that high-quality clinical trials in focal therapy are possible and should be encouraged; such trials have to be performed in a rigorous manner, relying on a clever trial design that employs the most modern tools and is likely to yield timely and clinically relevant results.
Future Directions
In the dynamic and constantly evolving field of prostate cancer, several important emerging concepts can be appreciated. It is likely that these notions will become standard practice in the future, pending sufficient supporting data.
FIGURE
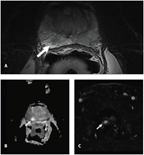
Multiparametric Magnetic Resonance Imaging (MRI) of a Highly Suspicious Focus (arrows) in the Posterior Medial and Lateral Peripheral Zone of the Prostate
One of the more important areas of innovation to emerge recently is the evolution of prostate imaging techniques. In the past, we relied almost exclusively on blind sampling of the prostate to evaluate and stage localized PCa. At present, several imaging techniques have been proposed that potentially allow for identification of the malignant foci within the gland. These methodologies include ultrasound-based techniques such as elastography, contrast-enhanced ultrasound, HistoScanning™, and acoustic radiation force impulse imaging.[19-21] In addition, MRI, specifically with the use of additional functional sequences (diffusion-weighted, contrast-enhanced spectroscopy), is considered to be the most solid imaging platform for localized PCa.[21,22] The Figure depicts MRI revealing a highly suspicious focus in the posterior medial and lateral peripheral zone that was pathologically confirmed to be a Gleason 7, organ-confined PCa.
The evolution of these imaging techniques has enabled us to further develop the concept of focal therapy on one hand, and to re-think the conventional paradigms of PCa management on the other. These modern imaging modalities are utilized in the diagnosis and staging of PCa, accurately targeting prostate biopsies to the individual lesion(s) rather than relying on blind sampling; in addition, they allow targeted treatment to be delivered to the malignant areas of the prostate.
With further confidence in the reliability of imaging, the status quo of blind biopsy sampling of the prostate could be overturned by increased use of image-guided targeted biopsies of the lesion itself. Furthermore, imaging is likely to become an integral part of active surveillance protocols,[23] both for initial staging and follow-up; in such a protocol, a reassuring imaging study, along with other clinical parameters, could reduce the number of surveillance biopsies and/or target these biopsies to specific lesions. This could potentially reduce biopsy-related morbidity and enhance the appeal of active surveillance protocols to the patient.
Summary
With the mounting evidence of the burden of effects from whole-gland treatment and, at the very least, inconclusive benefit of radical treatment in a significant portion of men diagnosed with prostate cancer, it is vital to devise strategies that minimize morbidity while offering oncological control of the disease.
We suggest that focal therapy and active surveillance are tightly associated with one another, being essentially parts of a shared approach for the management of prostate cancer that aims to safely avoid radical treatments and their side effects while providing oncologic control of PCa. More effort should be invested in defining the optimal use, indications, and protocols for these management strategies.
In conclusion, current data suggest that we need to become more comfortable with active surveillance and focal therapy as management strategies for localized prostate cancer. Through further understanding of prostate cancer biology, we need to determine the least invasive individualized management options without compromising cancer control outcomes. As new evidence shapes our management approaches, active surveillance and focal therapy are expected to assume key roles in the routine treatment of men with prostate cancer.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. de la Rosette J, Ahmed H, Barentsz J, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24:775-80.
2. Tsivian M, Polascik TJ. Prostate cancer: ideal candidates for focal therapy. Nat Rev Urol. 2012;9:12,13.
3. Marberger M, Barentsz J, Emberton M, et al. Novel approaches to improve prostate cancer diagnosis and management in early-stage disease. BJU Int. 2012;109(Suppl 2):1-7.
4. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203-13.
5. Wu GH, Auvinen A, Maattanen L, et al. Number of screens for overdetection as an indicator of absolute risk of overdiagnosis in prostate cancer screening. Int J Cancer. 2012;131:1367-75.
6. Kapoor DA, Zimberg SH, Ohrin LM, et al. Utilization trends in prostate cancer therapy. J Urol. 2011; 186:860-4.
7. Namiki S, Arai Y. Health-related quality of life in men with localized prostate cancer. Int J Urol. 2010;17: 125-38.
8. Bahn D, de Castro Abreu AL, Gill IS, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol. 2012;62:55-63.
9. Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13:622-32.
10. Tsivian M, Polascik TJ. Focal cryotherapy for prostate cancer. Curr Urol Rep. 2010;11:147-151.
11. Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126-31.
12. Abern MR, Tsivian M, Polascik TJ. Focal therapy of prostate cancer: evidence-based analysis for modern selection criteria. Curr Urol Rep. 2012;13:160-9.
13. Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361:1704-6.
14. Gulati R, Wever EM, Tsodikov A, et al. What if I don’t treat my PSA-detected prostate cancer? Answers from three natural history models. Cancer Epidemiol Biomarkers Prev. 2011;20:740-50.
15. Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430-9.
16. Si T, Guo Z, Hao X. Immunologic response to primary cryoablation of high-risk prostate cancer. Cryobiology. 2008;57:66-71.
17. Dall’era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012;62:976-83.
18. Palisaar JR, Noldus J, Loppenberg B, et al. Comprehensive report on prostate cancer misclassification by 16 currently used low-risk and active surveillance criteria. BJU Int. 2012;110:E172-81
19. Smeenge M, de la Rosette JJ, Wijkstra H. Current status of transrectal ultrasound techniques in prostate cancer. Curr Opin Urol. 2012;22:297-302.
20. Palmeri ML, Nightingale KR. Acoustic radiation force-based elasticity imaging methods. Interface Focus. 2011;1:553-64.
21. Hoang AN, Volkin D, Yerram NK, et al. Image guidance in the focal treatment of prostate cancer. Curr Opin Urol. 2012;22:328-35.
22. Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477-94.
23. Margel D, Yap SA, Lawrentschuk N, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187:1247-52.
24. Lambert EH, Bolte K, Masson P, Katz AE. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology. 2007; 69:1117-20.
25. Onik G, Vaughan D, Lotenfoe R, et al. “Male lumpectomy”: focal therapy for prostate cancer using cryoablation. Urology. 2007;70(6 Suppl):16-21.
26. Ahmed HU, Freeman A, Kirkham A, et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011;185:1246-54.
27. El Fegoun AB, Barret E, Prapotnich D, et al. Focal therapy with high-intensity focused ultrasound for prostate cancer in the elderly. A feasibility study with 10 years follow-up. Int Braz J Urol. 2011;37:213-19.