Expert Commentary on the Product Profile of Enfortumab Vedotin Plus Pembrolizumab
Kirollos S. Hanna, PharmD, BCPS, BCOP, FACCC, gives his perspective on the approval of enfortumab vedotin plus pembrolizumab for patients with urothelial carcinoma.
ONCOLOGY spoke with Kirollos S. Hanna, PharmD, BCPS, BCOP, FACC, about the recent approval of enfortumab vedotin plus pembrolizumab. He discussed how the trial has significantly impacted the bladder cancer space and the improvement it has made to the standard of care.
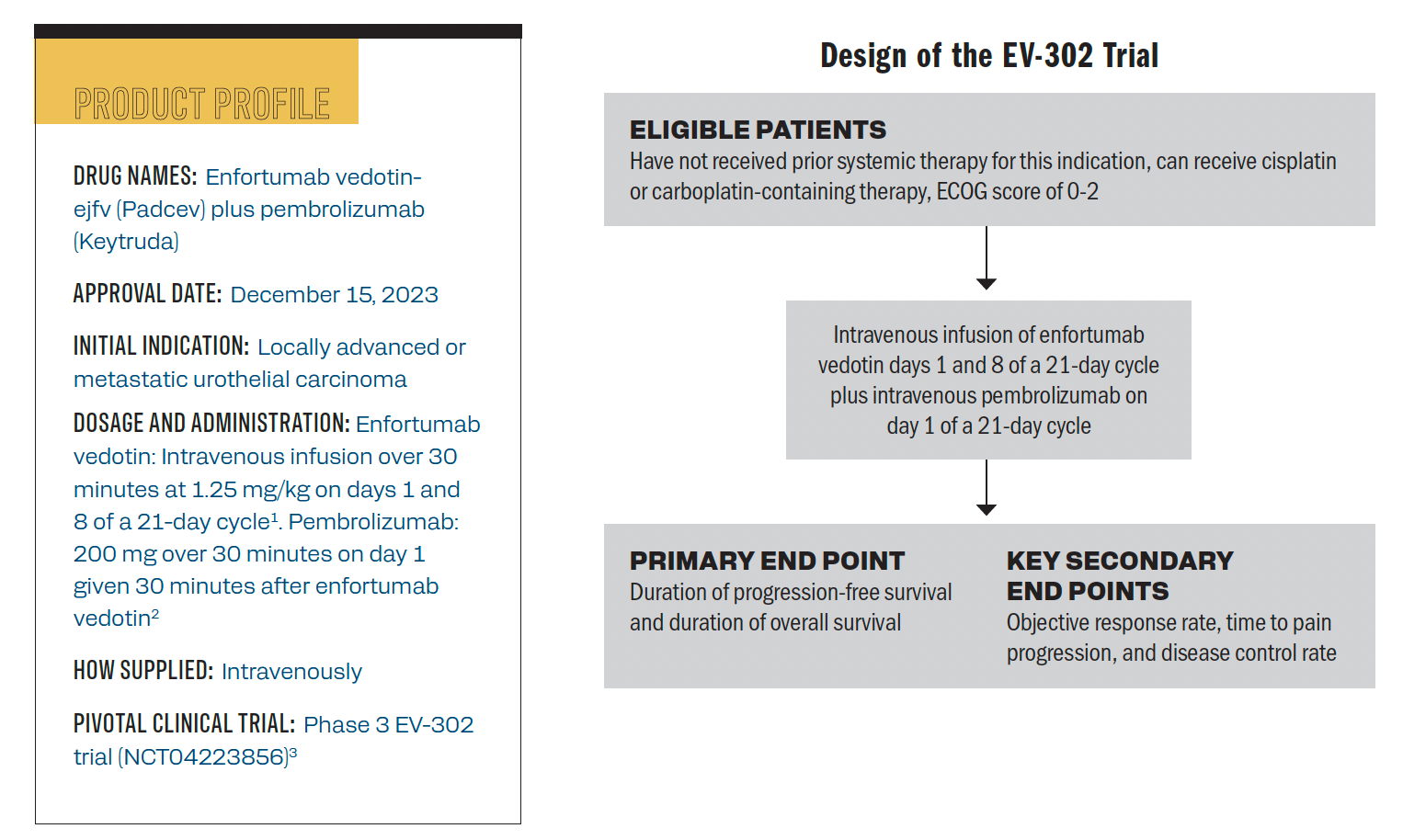
The product profile of enfortumab vedotin and trial design of the phase 3 EV-302 trial.
Can you summarize the recent FDA approval of enfortumab vedotin plus pembrolizumab for patients with locally advanced or metastatic urothelial cancer?
Hanna: For patients with bladder cancer, this was an exciting approval from the FDA. When you look at the numerous advances that we’ve seen in bladder cancer over the years, we’ve known that enfortumab vedotin has a role in patients with refractory or relapsed disease. We’ve also seen pembrolizumab bringing some benefits. The EV-302 study was exciting because it originally provided an accelerated approval for this patient population; there was the phase 1/2 EV-103 data [NCT03288545], and phase 3 EV-302 [NCT04223856] was the confirmatory study.4
Prior to the official FDA approval when the data were presented at the European Society for Medical Oncology [ESMO], this study received a standing ovation.5 It was exciting just to see the benefit that this brings to those patients in the frontline [setting] with metastatic bladder cancer because we haven’t seen anything in a long time. That has, I would say, displaced standard cytotoxic chemotherapy.
When you look at EV-302, this was a randomized trial, with almost 900 patients. They had no prior systemic therapy, and they were either [randomly assigned] to pembrolizumab with enfortumab vedotin or put on standard cisplatin, platinum-based chemotherapy. We saw a significant benefit in the overall survival for the FDA approval: It was about a 31-and-a-half-month overall survival benefit with the combination [arm] vs only about 16 months in the patients who received platinum-based chemotherapy. This was something very, very significant—a lot of exciting things going on within the space. The National Comprehensive Cancer Network has placed this with some solid recommendations, what we saw come out of ESMO. Now with the FDA having granted accelerated approval, [there are] a lot of exciting things that we’ve seen with the bladder [cancer] population.
What is the specific patient population that may be treated with this FDA-approved regimen?
Hanna: When you look at the patient population, there’s not a unique patient who would benefit more than the other. When you look at the intent-to-treat population, [investigators] looked within the clinical trial based on various stratification criteria, they looked at the degree of PD-L1 expression, they looked at the degree of NECTIN4 expression. Regardless of that, these patients [showed a] benefit, so all comers will benefit from this combination therapy. When you look at this patient population, a lot of times in that frontline setting, we start to ask ourselves, “Is this patient platinum eligible or platinum ineligible? Could they receive cisplatin? Should they receive carboplatin?” This [approval] takes that out of the equation. Given the combination therapy here, with this particular regimen, there’s no particular expression that’s needed.
An area where we’re still learning a little bit more is those with muscle-invasive bladder cancer. These patients are still being treated with neoadjuvant chemotherapy, things like dose-dense MVAC [methotrexate, vinblastine, Adriamycin, and cisplatin], or a radical cystectomy. In some subsets of patients, they might receive adjuvant nivolumab [Opdivo]. If for some reason I see a unique patient who is on adjuvant nivolumab, in that muscle-invasive setting, and then progresses quickly, that might be a patient I might be a little bit hesitant to treat with the enfortumab vedotin plus pembrolizumab combination. We know they have just progressed on immunotherapy and quickly, so they might not get that maximum benefit from [the combination] therapy. While enfortumab vedotin will still be a very effective agent, that might be a patient I would consider maybe a different approach, depending on how long it’s been from their chemotherapy administration.
We’ve seen a lot of exciting things move in this space as well. Just recently, we had nivolumab approved with combination chemotherapy, but then again, if [the patients] progressed fairly quickly on nivolumab, they wouldn’t be an eligible candidate [for this combination treatment].6
What are some safety considerations or any adverse effects that are associated with this combination?
Hanna: When we look at the safety, we now have 2 therapeutics that are going to come with some shared [adverse] effects [AEs] in some regard, but also significant differences when you look at enfortumab vedotin; it is cytotoxic in nature, while it is targeting NECTIN4 expression. It is cytotoxic through its payload, so we’re going to be considering cytotoxic AEs. When you look at pembrolizumab, it’s immunotherapy, and you’re going to be worried about those immune-related AEs [IRAEs] that we’re all very familiar with. When you talk about enfortumab vedotin, 3 things come to mind around AEs.
We need to be monitoring, and we should be vigilant of neuropathy whether a patient has preexisting neuropathy or not; the MMAE component is known to lead to neuropathy. It’s generally sensory, but it does get better with dose reductions and dose modifications. We have to watch that in our patients. No. 2 is that with enfortumab vedotin, some of these patients might experience a rash, and in the clinical studies, about half of the patients experienced a rash. In rare cases, it could be a severe rash. It could be things like Stevens-Johnson syndrome. The reason for that is because we know there is NECTIN4 expression that is on the skin. As these patients are coming in for their infusion, whether you’re using enfortumab vedotin for them as monotherapy or in combination with pembrolizumab, because there are some differences in the schedule, these patients will be coming in roughly every week. There’s a lot of frequent touch points with these patients. When they are seeing our providers or when they are in the infusion center, we want to make sure that we are looking at their skin and having these evaluations. Don’t just look at their extremities: Look at their chest, look at their back, and make sure that that rash, we want to catch it early vs getting to a severe stage. Again, that improves with dose holds and dose modifications, and supportive care as well.
The other thing that comes to mind with enfortumab vedotin is going to be hyperglycemia. Hyperglycemia can sometimes occur with this. We just want to monitor it. If you have a patient who is diabetic, maybe monitor it a little bit more closely. It can exacerbate the neuropathy if it’s left uncontrolled, but just something to be cognizant of. Then, just being cytotoxic, you’re going to want to watch out for any hematologic AEs as you would with any other cytotoxic agent. The pembrolizumab is going to [result in] IRAEs. You have your standard IRAEs with the gastrointestinal AEs in your liver with alanine transaminase/aspartate transaminase elevations, transaminase impact, and skin toxicities.
The biggest thing is that skin AE I mentioned may be an immunotherapy-mediated rash, and you’re not sure whether is it due to pembrolizumab or to enfortumab vedotin. Enfortumab vedotin tends to be a little bit more blistering, cracking the skin dry, and very rough vs the [immunotherapy] at a low grade might just be a small rash on the skin that’s easy to manage. These are things we want to be cognizant of, the differences between the 2.
How does the efficacy of the combination compare with that of others in the space?
Hanna: This is the very first study that has demonstrated a significant improvement outside of the standard of care for frontline metastatic bladder cancer prior to this study. One thing that does come to mind is the phase 3 JAVELIN Bladder 100 study [NCT02603432].7 We haven’t seen anything exciting in bladder cancer come in that frontline setting that I recall prior to JAVELINE Bladder for a long time. JAVELIN Bladder treated patients with platinum chemotherapy and then put them on avelumab [Bavencio] switch or maintenance therapy. That was one of the very first studies that enhanced the response in the frontline setting where immunotherapy demonstrated an improvement in overall survival over observation or best supportive care. That’s what it was compared with.
We can look at EV-302, [where] you have these 2 drugs that have an 11- to 12-month overall survival benefit over platinum-containing chemotherapy. That is huge in terms of an efficacy perspective. We’re all also familiar with these agents. We know that they are active in subsequent lines, as well. It was nice to see that combination.
In contrast to that, the FDA approved the combination of nivolumab with cisplatin and gemcitabine. The benefit there, and while I don’t want to cross-trial compare, these weren’t compared head-to-head, with these regimens, the overall survival benefit that we saw from nivolumab plus cisplatin and gemcitabine wasn’t as significant as the difference we saw from pembrolizumab plus enfortumab vedotin. It will be interesting to see how [clinicians] leverage these, but the good thing about that indication is that if you do have that patient, for example, with preexisting neuropathy, and we’re concerned about administering [enfortumab vedotin like] we now know, you can add nivolumab to cisplatin and gemcitabine, and then evaluate how your patient is going to be doing on that.
One challenge outside of the EV-302 study is the placement of immunotherapy. If you’re [administering] platinum-containing chemotherapy, is it platinum-containing chemotherapy followed by avelumab maintenance a better approach? Or is the combination of nivolumab with platinum-containing chemotherapy a better approach all up front vs some sort of maintenance therapy? It’ll be interesting to see how as the data continue to be followed and mature, how that pans out. Over 30 to 40 years, we have not seen something improve upon the overall survival benefit as drastically as we’ve seen from EV-302.
What are some logistical considerations needed to administer this treatment?
Hanna: From a pembrolizumab standpoint, there’s not too much to consider. The dosing of pembrolizumab is agnostic. Whether you’re a 3-week institution or a 6-week institution, or you switch your patient at some point, there is nothing nuanced there. From an administration standpoint, as you’re building out treatment plans and treatment protocols, it’s important to highlight that the dosing of enfortumab vedotin monotherapy is different than the dosing of enfortumab vedotin combination. The enfortumab vedotin monotherapy is a 28-day regimen, given on days 1, 8, and 15 of a 28-day cycle. The dosing of enfortumab vedotin with pembrolizumab, because it has to be [administered] at a 3-week interval, it’s given on days 1 and 8 of a 21-day cycle. Regardless of how you’re dosing the pembrolizumab, that’s going to be something to be considered or just important to call out. The dose basis is still the same; you’re doing 1.25 mg/kg.
A couple of other things that I would say operationally are important to consider as well. You might want to work the antinausea prophylaxis into your [treatment plan]. The package insert on the labeling doesn’t recommend anything. In particular we see about a 40% emesis potential with enfortumab vedotin. It is generally low-grade, though. Having some type of antiemetics on board as a premedication or even as a take-home for patients is going to be important.
The other component from a pharmacy lens and operationally is we’ve gotten a lot of questions on...the enfortumab vedotin vial size, which is 20-mg and 300-mg vial sizes. The dose is 1.25 mg/kg, and you cap your patient at 100 kg. A lot of our antibody-drug conjugates are capped at maximum weight. If you do have a patient who is over 100 kg, which is a little bit rare in bladder cancer, especially if these patients have been heavily pretreated before, you don’t get a lot of patients who are 100 kg or above. But if you do, your dose of 1.25, some like to round it down to 1.20. They then have exact vial sizes.
Within the operations given, that these patients are coming in on day 8 or days 8 and 15 depending on how you’re leveraging enfortumab vedotin, having [strategies] built within your electronic medical records or education to staff to try to catch these AEs that we’ve highlighted throughout this discussion [is recommended]. Sometimes these patients on days 8 and 15 aren’t even seeing a provider, they’re just coming into the infusion center, maybe getting their labs checked. As long as their labs meet the parameters, we continue to treat them. What does their neuropathy look like, are we assessing for that? Do we know what the rash looks like? Are they having skin toxicities that they’re not reporting or we’re not capturing? That will help in terms of patients being on therapy for as long as possible as long as they’re benefiting.
Are there any specific monitoring requirements for patients who receive enfortumab vedotin plus pembrolizumab?
Hanna: The key monitoring parameters here outside of the things we highlighted in terms of the AEs, you want to be watching your metabolic panels. Enfortumab vedotin could still have some type of hepatic toxicity associated with it, but also, because these patients are on pembrolizumab you could have some transaminase elevation from the immunotherapy component.
We also want to be monitoring the glucose. Some will tell you as you’re monitoring glucose, to have a baseline [hemoglobin] A1c might be valuable to you. Some [clinicians] don’t want to react off of a single glucose reading. They don’t always have that glucose of 250 mg/dL be the sole cutoff, and they just watch trends with patients. Others will react based on that.
Of course, your complete blood count [should also be monitored]. [Enfortumab vedotin] is cytotoxic. In nature, we want to make sure that we’re assessing what is clinically appropriate for our patients. Those are generally the key things that we’re going to be monitoring for this population. The only other thing is for the pembrolizumab, getting your baseline thyroid function, and assessing that and addressing that as needs may come up.
From an oncology pharmacist perspective, how can this approval impact the treatment landscape for the specific patient population?
Hanna: Oncology pharmacists play a critical role across the board, whether we’re talking about large academic or community practice. It just depends on how that pharmacist is plugged into the model within the practice. Education is something that pharmacists excel at. Whether it’s education around the therapy, the supportive care, pharmacists can certainly help their AE monitoring and support when AEs do come up. In addition to that, when these patients are coming into that infusion center and not seeing a provider, the clinical pharmacist can certainly be there to help with lab evaluation, counseling, or addressing things with our nursing colleagues as well.
The other component of it is this regimen and how it’s going to shift the disease paradigm. If pharmacists are functioning on a pharmacy and therapeutics committee and doing the analysis of how the organization wants to operationalize this, this regimen certainly does come with a cost. You’re combining 2 expensive therapeutics that are going to increase the total cost of care for patients, particularly in the frontline setting. Even when you look at the 2 or 3 lines of therapy for bladder cancer, these patients are still going to be exposed to these therapeutics at one point in time, whether you leverage this on the front line, or whether you do platinum therapy, then they go on immunotherapy, then they go on enfortumab vedotin. From a bladder cancer perspective in total, it doesn’t increase the cost of care, but it shifts the cost of care to that frontline setting, given the clinical efficacy of the profile of the EV-302 study. I would encourage people to still leverage the pharmacy for awareness that clinical trials are still strongly encouraged in bladder cancer, and metastatic bladder cancer across all lines of therapy because we still have a significant way to go in terms of making an impact. We’ve seen a lot of novel therapeutics within this space. When you look at the 5-year survival, it’s only about 8% to 10%. It’s still a far ways from where we would like to be for this patient population.
References
- Padcev. Prescribing information. Astellas; 2023. Accessed March 20, 2024. https://shorturl.at/uyIO1
- Keytruda. Prescribing information. Merck; 2023. Accessed March 20, 2024. https://shorturl.at/fhjX7
- FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. FDA. December 15, 2023. Accessed March 20, 2024. https://bit.ly/48ls9bi
- Hoimes CJ, Flaig TW, Milowsky MI, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. 2023;41(1):22-31. doi:10.1200/JCO.22.01643
- Powels TB, Perez Valderrama B, Gupta S, et al. EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Annals of Oncol. 2023;34(suppl 2):S1281-S1282. doi:10.1016/S0923-7534(23)X0011-8
- FDA approves nivolumab in combination with cisplatin and gemcitabine for unresectable or metastatic urothelial carcinoma. FDA. March 6, 2024. Accessed March 20, 2024. https://shorturl.at/jtwL9
- Powles T, Park SH, Caserta C, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-3492. doi:10.1200/JCO.22.01792
