Expert Commentary on the Product Profile of Tisotumab Vedotin in Cervical Cancer
Laura Bucher-Bailey, PharmD, discussed the approval of tisotumab vedotin-tftv for patients with recurrent or metastatic cervical cancer who have had progression after chemotherapy.
Laura Bucher-Bailey, PharmD

Laura Bucher-Bailey, PharmD, discussed the approval of tisotumab vedotin-tftv (Tivdak) for patients with recurrent or metastatic cervical cancer who have had progression after chemotherapy. Bailey discussed the benefit the treatment brings to patients and how it compares with other options in the space when
addressing adverse effects (AEs).
Product profile of tisotumab vedotin

Q / What is the mechanism of action of tisotumab vedotin?
Bailey / It is an antibody-drug conjugate. It’s made up of 3 components. It has the tisotumab, a cleavable linker, and then MMAE. Tisotumab is an antibody that looks for tissue factor antigens on the surface of cells. The MMAE is a microtubule-disrupting agent, so it prevents cells from dividing, which leads
to apoptosis.
Q / Which patients are most likely to benefit from this treatment?
Bailey / It’s indicated for adults with recurrent metastatic cervical cancer. It’s approved for second-line treatment, so they would have had to have progressed on first-line treatment. There are 2 other preferred agents as second-line [options]. Pembrolizumab [Keytruda] is preferred for patients with certain tumor markers, and then tisotumab vedotin and cemiplimab-rwlc [Libtayo] are also second-line options. In our practice, what I’ve been seeing is they’re using it more as third- or fourth-line therapy after patients progress on the first line. Then they’ve been using pembrolizumab. Now we’re seeing tisotumab vedotin being considered.
Design of the phase 3 innovaTV 301 trial
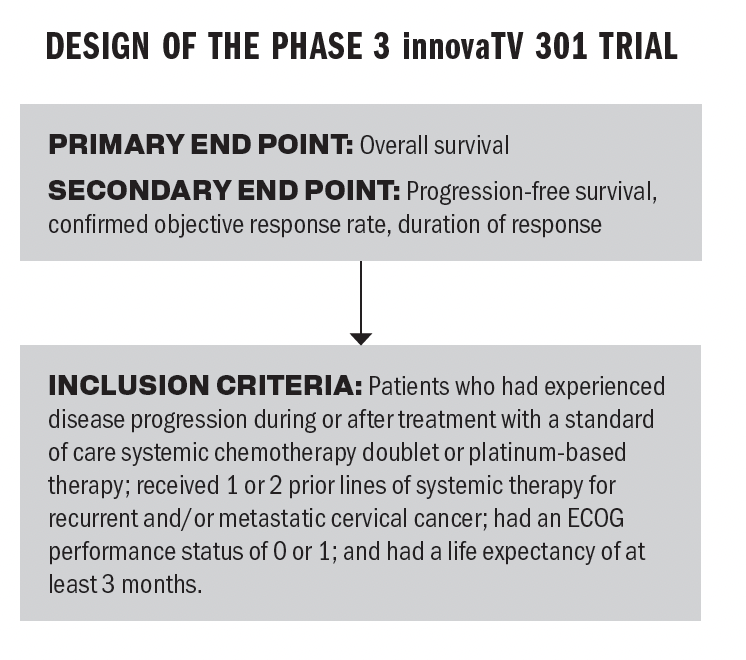
Q / The innovaTV 301 trial demonstrated an improvement in overall survival [OS]. How significant is this improvement in the context of other treatment options in the space?
Bailey / An improvement in OS is what we’re looking for. You want patients to survive a little longer. The treatment options for patients who have recurrent cervical cancer are not super effective. It’s always nice to have new options with new mechanisms of action. The leading OS from the trial [was] an 11.5-month OS in patients who received tisotumab vedotin vs 9.5 months [who received chemotherapy]. That’s 2 more months of OS, and that difference is a 30% lower risk of death in the patients who received tisotumab. Anytime you increase OS, that’s a step in the right direction.
Q / What are some of the most common AEs associated with tisotumab vedotin, and how do they compare with other treatments in the space?
Bailey / The most common AEs are ocular toxicities, peripheral neuropathy, and hemorrhage. Some patients also experience pneumonitis and cutaneous reactions but to a lesser degree. Immunotherapy, in general, is associated with an increased risk of immune-mediated AEs. The other second-line options also have pneumonitis and other immune-mediated AEs associated with them. So that’s similar.
For tisotumab vedotin, the big thing to point out is that there is a black box warning regarding ocular toxicity, and there are also ocular care requirements. Prior to initiating treatment, the patient needs to be examined by an ophthalmologist. They will be prescribed 3 types of eye drops: a corticosteroid, a vasoconstrictor, and lubricating eye drops. They have to bring those with them to treatment. They need to start before treatment and then…they need to place cold packs on their eyes throughout the duration of treatment. They’re not allowed to wear contact lenses throughout the duration of treatment. For some patients, that might be a consideration. The other second-line options of pembrolizumab and cemiplimab don’t have the same ocular toxicity. That’s a big difference between tisotumab and the other second-line agents.
Q / What are some of the potential resistant mechanisms associated with this treatment?
Bailey / With immunotherapy, resistance does tend to develop. With an
antibody-drug conjugate, specifically, you can develop resistance to the antibody portion or the payload portion. If the issue is antibody related, but they still want to get the MMAE into the cell, they can change what they’re targeting on the cell surface to still get the payload into the cell. If the problem is the opposite, then the cell is either kicking the MMAE out or changing the way it processes it. But the cell is still taking up the antibody; the antibody is still recognizing the antigen on the outside of the cell. They can change the payload and get a new microtubule-disrupting agent inside. It’s so cool that we can find antigens on the cells of tumors, target those, and then get something inside that will be super effective but won’t affect cells globally. Then you don’t have all those AEs that you see with the more traditional chemotherapy, [such as] nausea, vomiting, mouth sores, and hair loss. It’s amazing that we’re figuring these things out.
Q / Where do you see this agent headed?
Bailey / There are some trials right now looking at tisotumab vedotin with other solid tumors, [such as in] metastatic/recurrent, pancreatic and colorectal cancer, other solid tumors that have tissue factor protein on the cell surface. Anytime you have a new mechanism of action, a new opportunity to use a new class of drugs in a patient who has recurrent disease, it’s always an improvement.
When I was looking at the subgroup analysis, I thought that it was very interesting that the patients who had received bevacizumab [Avastin] prior to tisotumab did better with tisotumab vedotin than investigator’s choice chemotherapy, but for patients who did not receive bevacizumab, there was no difference between the patients who got tisotumab vedotin [vs] investigator’s choice chemotherapy. The investigator does mention in the results that there’s no biological reason for the differing outcomes of subsequent therapy in patients who have received bevacizumab previously vs those who have not. That’s super interesting and something to look into.
Q / Is there anything else you would like to highlight?
Bailey / The dose of tisotumab vedotin is 2 mg/kg up to a maximum of 200 mg. Any patient [weighing more than] 100 kg would still just get 200 mg. It’s 30 minutes every 3 weeks, just like the other 2 second-line options. This agent does require the cold packs and all the eye drops, and the package insert has a nice outline of dosage adjustments for patients experiencing AEs. Also, the use of tisotumab vedotin with any strong CYP3/4 inhibitors will increase the patient’s exposure to MMAE. That also increases the patient’s risk of AEs, and then you should avoid the use of tisotumab vedotin in patients with moderate to severe hepatic impairment. [The trial] did see that patients with mild to moderate hepatic impairment had, about a 37% increased exposure to MMAE, so they didn’t even use it. They didn’t use it in patients with moderate or severe hepatic impairment. It’s recommended that you avoid use in those patients.
References
- FDA approves tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. News release. FDA. April 29, 2024. Accessed August 15, 2024. https://shorturl.at/W4M69
- Tivdak. Prescribing information. Seagen; 2024. Accessed August 15, 2024. https://shorturl.at/kcaKv
- Slomovitz BM, Gonzalez Martin A, Fujiwara K, et al. innovaTV 301/ENGOT-cx12/GOG-3057: a global, randomized, open-label, phase 3 study of tisotumab vedotin vs investigator’s choice of chemotherapy in 2L or 3L recurrent or metastatic cervical cancer. Presented at 2023 Annual Global Meeting of the International Gynecologic Cancer Society; November 5-7, 2023; Seoul, Korea. Abstract SE006/1616
